Evaluation of the Effect of Lorlatinib on CYP2B6, CYP2C9, UGT, and P-Glycoprotein Substrates in Patients with Advanced Non-Small Cell Lung Cancer
- PMID: 38079095
- PMCID: PMC10847213
- DOI: 10.1007/s40262-023-01309-4
Evaluation of the Effect of Lorlatinib on CYP2B6, CYP2C9, UGT, and P-Glycoprotein Substrates in Patients with Advanced Non-Small Cell Lung Cancer
Abstract
Background and objective: Lorlatinib is a tyrosine kinase inhibitor approved for the treatment of advanced anaplastic lymphoma kinase-positive non-small cell lung cancer. This study assessed the effect of steady-state lorlatinib on the metabolic enzymes cytochrome P450 (CYP) 2B6, CYP2C9, and uridine 5'-diphospho-glucuronosyltransferase (UGT) and the P-glycoprotein (P-gp) transporter.
Methods: Thirty-two patients received a single oral dose of a probe drug on Day - 2 to determine the pharmacokinetics of the probe drug alone. Starting on Day 1, patients received 100 mg oral lorlatinib daily. On Day 15, a single oral dose of the probe drug was administered concurrently with lorlatinib. Pharmacokinetic parameters for these probe substrates were assessed.
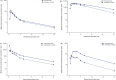
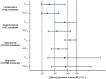
Results: Plasma exposures of all probe substrates were reduced by lorlatinib compared with the probe alone. The greatest reduction in area under the plasma concentration-time curve from time zero to infinity (AUC∞) and maximum (peak) plasma drug concentration (Cmax) (67% and 63% decrease, respectively) was observed with the P-gp probe substrate fexofenadine. Lorlatinib coadministration also decreased the AUC∞ and Cmax of bupropion (CYP2B6 probe substrate) by 25% and 27%, tolbutamide (CYP2C9 probe substrate) by 43% and 15%, and acetaminophen (UGT probe substrate) by 45% and 28%, respectively.
Conclusions: Lorlatinib is a net moderate inducer of P-gp and a weak inducer of CYP2B6, CYP2C9, and UGT after steady state is achieved with daily dosing. Medications that are P-gp substrates with a narrow therapeutic window should be avoided in patients taking lorlatinib; no dose modifications are needed with substrates of CYP2B6, CYP2C9, or UGT.
Clinicaltrials: gov: NCT01970865.
© 2023. The Author(s).
Conflict of interest statement
JC was employed by Pfizer at the time of the work, owns stocks in Pfizer, and is currently employed by Roche/Genentech. AB reports advisory board fees from AstraZeneca, Bristol Myers Squibb, Pfizer, and Roche; speaker fees from Eli Lilly, Pfizer, and Roche. D-WK reports administrative support and grants from Amgen, AstraZeneca, Boehringer Ingelheim, Bridge BioTherapeutics, Chong Keun Dang, Daiichi Sankyo, GlaxoSmithKline, Janssen, Merck, Merus, MSD, Novartis, Pfizer, Roche, Takeda, and Yuhan; grants from Alpha Biopharma, Hanmi, InnoN, Mirati Therapeutics, ONO Pharmaceutical, Turning Point Therapeutics, and Xcovery. HM reports advisory board fees from AstraZeneca, Genentech, and Zentalis; received a grant from U CAN-CER VIVE Foundation; and received institutional research funding from Pfizer. JB reports consulting fees or honoraria from BeiGene, Blueprint Medicines, Eli Lilly, Janssen, Merck, Mirati, Pfizer, and Turning Point Therapeutics. RC declares no conflicts of interest. S-HIO reports consulting fees or honorarium from AnHeart Therapeutics, BeiGene, Daiichi Sankyo, Eli Lilly, Johnson and Johnson/Janssen, and Pfizer; speaker fees from DAVA Oncology, Johnson and Johnson/Janssen, and Pfizer; advisory board fees from Elevation Oncology; stocks in Elevation Oncology and Turning Point Therapeutics. BJS reports advisory board fees or honoraria from Amgen, AstraZeneca, Bristol Myers Squibb, Merck, Pfizer, Roche/Genentech; and received fees from BeiGene, Janssen, Novartis, and Takeda. RAS reports advisory board fees or honoraria from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, EMD Serono, J INTS BIO, Janssen, Merck, Microquin, Novartis, Pfizer, Puma Biotechnology, Roche, Taiho, Takeda, ThermoFisher, and Yuhan; and research grants from AstraZeneca and Boehringer Ingelheim. EF reports consulting fees or honoraria from Amgen, AstraZeneca, BerGenBio, Bristol Myers Squibb, Daichi Sankyo, Eli Lilly, F. Hoffman-La Roche, GlaxoSmithKline, Janssen, Merck Serono, MSD, Novartis, Peptomyc, Pfizer, Sanofi, and Takeda; speaker fees from Amgen, AstraZeneca, Bristol Myers Squibb, Eli Lilly, F. Hoffman-La Roche, Janssen, Medical Trends, MedScape, MSD, Novartis, PeerVoice, Pfizer, Sanofi, Takeda, and Touch Oncology; and is an independent member of the board at Grifols. AS is employed by and owns stock in Novartis. HT, JSC, KL, MO, CT, and YKP are employed by and own stocks in Pfizer.
Figures
References
-
- Johnson TW, Richardson PF, Bailey S, Brooun A, Burke BJ, Collins MR, et al. Discovery of (10R)-7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8,4-(m etheno)pyrazolo[4,3-h][2,5,11]-benzoxadiazacyclotetradecine-3-carbonitrile (PF-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. J Med Chem. 2014;57(11):4720–4744. doi: 10.1021/jm500261q. - DOI - PubMed
-
- Shaw AT, Felip E, Bauer TM, Besse B, Navarro A, Postel-Vinay S, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017;18(12):1590–1599. doi: 10.1016/S1470-2045(17)30680-0. - DOI - PMC - PubMed
-
- Pfizer Inc. LORBRENA® (lorlatinib): Prescribing Information. 2021 [cited 2021 August 10]. http://labeling.pfizer.com/ShowLabeling.aspx?id=11140.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

