PAK2 is necessary for myelination in the peripheral nervous system
- PMID: 38079473
- PMCID: PMC11068108
- DOI: 10.1093/brain/awad413
PAK2 is necessary for myelination in the peripheral nervous system
Abstract
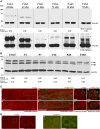
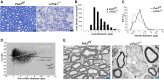
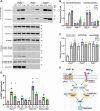
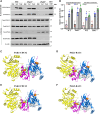
Myelination enables electrical impulses to propagate on axons at the highest speed, encoding essential life functions. The Rho family GTPases, RAC1 and CDC42, have been shown to critically regulate Schwann cell myelination. P21-activated kinase 2 (PAK2) is an effector of RAC1/CDC42, but its specific role in myelination remains undetermined. We produced a Schwann cell-specific knockout mouse of Pak2 (scPak2-/-) to evaluate PAK2's role in myelination. Deletion of Pak2, specifically in mouse Schwann cells, resulted in severe hypomyelination, slowed nerve conduction velocity and behaviour dysfunctions in the scPak2-/- peripheral nerve. Many Schwann cells in scPak2-/- sciatic nerves were arrested at the stage of axonal sorting. These abnormalities were rescued by reintroducing Pak2, but not the kinase-dead mutation of Pak2, via lentivirus delivery to scPak2-/- Schwann cells in vivo. Moreover, ablation of Pak2 in Schwann cells blocked the promyelinating effect driven by neuregulin-1, prion protein and inactivated RAC1/CDC42. Conversely, the ablation of Pak2 in neurons exhibited no phenotype. Such PAK2 activity can also be either enhanced or inhibited by different myelin lipids. We have identified a novel promyelinating factor, PAK2, that acts as a critical convergence point for multiple promyelinating signalling pathways. The promyelination by PAK2 is Schwann cell-autonomous. Myelin lipids, identified as inhibitors or activators of PAK2, may be utilized to develop therapies for repairing abnormal myelin in peripheral neuropathies.
Keywords: Pak2 knock-out mouse; GTPases Rac1/Cdc42; Schwann cells; myelin lipids; myelination; peripheral nerve.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

