Application of ZnO Nanoparticles Encapsulated in Mesoporous Silica on the Abaxial Side of a Solanum lycopersicum Leaf Enhances Zn Uptake and Translocation via the Phloem
- PMID: 38079531
- PMCID: PMC10753877
- DOI: 10.1021/acs.est.3c06424
Application of ZnO Nanoparticles Encapsulated in Mesoporous Silica on the Abaxial Side of a Solanum lycopersicum Leaf Enhances Zn Uptake and Translocation via the Phloem
Abstract
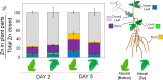
Foliar application of nutrient nanoparticles (NPs) is a promising strategy for improving fertilization efficiency in agriculture. Phloem translocation of NPs from leaves is required for efficient fertilization but is currently considered to be feasible only for NPs smaller than a cell wall pore size exclusion limit of <20 nm. Using mass spectrometry imaging, we provide here the first direct evidence for phloem localization and translocation of a larger (∼70 nm) fertilizer NP comprised of ZnO encapsulated in mesoporous SiO2 (ZnO@MSN) following foliar deposition. The Si content in the phloem tissue of the petiole connected to the dosed leaf was ∼10 times higher than in the xylem tissue, and ∼100 times higher than the phloem tissue of an untreated tomato plant petiole. Direct evidence of NPs in individual phloem cells has only previously been shown for smaller NPs introduced invasively in the plant. Furthermore, we show that uptake and translocation of the NPs can be enhanced by their application on the abaxial (lower) side of the leaf. Applying ZnO@MSN to the abaxial side of a single leaf resulted in a 56% higher uptake of Zn as well as higher translocation to the younger (upper) leaves and to the roots, than dosing the adaxial (top) side of a leaf. The higher abaxial uptake of NPs is in alignment with the higher stomatal density and lower density of mesophyll tissues on that side and has not been demonstrated before.
Keywords: Nanofertilizer; ZnO nanoparticles; foliar application; mesoporous silica nanocarriers; phloem translocation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Willett W.; Rockström J.; Loken B.; Springmann M.; Lang T.; Vermeulen S.; Garnett T.; Tilman D.; DeClerck F.; Wood A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on Healthy Diets from Sustainable Food Systems. Lancet 2019, 393 (10170), 447–492. 10.1016/S0140-6736(18)31788-4. - DOI - PubMed
-
- Hofmann T.; Lowry G. V.; Ghoshal S.; Tufenkji N.; Brambilla D.; Dutcher J. R.; Gilbertson L. M.; Giraldo J. P.; Kinsella J. M.; Landry M. P. Technology Readiness and Overcoming Barriers to Sustainably Implement Nanotechnology-Enabled Plant Agriculture. Nat. Food. 2020, 1 (7), 416–425. 10.1038/s43016-020-0110-1. - DOI
-
- Hafeez B.; Khanif Y. M.; Saleem M. Role of Zinc in Plant Nutrition-a Review. Journal of Experimental Agriculture International 2013, 3 (2), 374–391. 10.9734/AJEA/2013/2746. - DOI
-
- Tscharntke T.; Clough Y.; Wanger T. C.; Jackson L.; Motzke I.; Perfecto I.; Vandermeer J.; Whitbread A. Global Food Security, Biodiversity Conservation and the Future of Agricultural Intensification. Biol. Conserv. 2012, 151 (1), 53–59. 10.1016/j.biocon.2012.01.068. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

