Microbially induced precipitation of silica by anaerobic methane-oxidizing consortia and implications for microbial fossil preservation
- PMID: 38079551
- PMCID: PMC10743459
- DOI: 10.1073/pnas.2302156120
Microbially induced precipitation of silica by anaerobic methane-oxidizing consortia and implications for microbial fossil preservation
Abstract
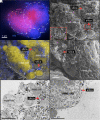
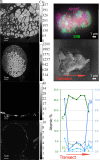
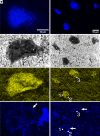
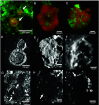
Authigenic carbonate minerals can preserve biosignatures of microbial anaerobic oxidation of methane (AOM) in the rock record. It is not currently known whether the microorganisms that mediate sulfate-coupled AOM-often occurring as multicelled consortia of anaerobic methanotrophic archaea (ANME) and sulfate-reducing bacteria (SRB)-are preserved as microfossils. Electron microscopy of ANME-SRB consortia in methane seep sediments has shown that these microorganisms can be associated with silicate minerals such as clays [Chen et al., Sci. Rep. 4, 1-9 (2014)], but the biogenicity of these phases, their geochemical composition, and their potential preservation in the rock record is poorly constrained. Long-term laboratory AOM enrichment cultures in sediment-free artificial seawater [Yu et al., Appl. Environ. Microbiol. 88, e02109-21 (2022)] resulted in precipitation of amorphous silicate particles (~200 nm) within clusters of exopolymer-rich AOM consortia from media undersaturated with respect to silica, suggestive of a microbially mediated process. The use of techniques like correlative fluorescence in situ hybridization (FISH), scanning electron microscopy with energy dispersive X-ray spectroscopy (SEM-EDS), and nanoscale secondary ion mass spectrometry (nanoSIMS) on AOM consortia from methane seep authigenic carbonates and sediments further revealed that they are enveloped in a silica-rich phase similar to the mineral phase on ANME-SRB consortia in enrichment cultures. Like in cyanobacteria [Moore et al., Geology 48, 862-866 (2020)], the Si-rich phases on ANME-SRB consortia identified here may enhance their preservation as microfossils. The morphology of these silica-rich precipitates, consistent with amorphous-type clay-like spheroids formed within organic assemblages, provides an additional mineralogical signature that may assist in the search for structural remnants of microbial consortia in rocks which formed in methane-rich environments from Earth and other planetary bodies.
Keywords: ANME-SRB; amorphous silica; methane seeps; microbial biomineralization; microfossils.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

Similar articles
-
Community Structure and Microbial Associations in Sediment-Free Methanotrophic Enrichment Cultures from a Marine Methane Seep.Appl Environ Microbiol. 2022 Jun 14;88(11):e0210921. doi: 10.1128/aem.02109-21. Epub 2022 May 23. Appl Environ Microbiol. 2022. PMID: 35604226 Free PMC article.
-
Experimentally-validated correlation analysis reveals new anaerobic methane oxidation partnerships with consortium-level heterogeneity in diazotrophy.ISME J. 2021 Feb;15(2):377-396. doi: 10.1038/s41396-020-00757-1. Epub 2020 Oct 15. ISME J. 2021. PMID: 33060828 Free PMC article.
-
Subgroup Characteristics of Marine Methane-Oxidizing ANME-2 Archaea and Their Syntrophic Partners as Revealed by Integrated Multimodal Analytical Microscopy.Appl Environ Microbiol. 2018 May 17;84(11):e00399-18. doi: 10.1128/AEM.00399-18. Print 2018 Jun 1. Appl Environ Microbiol. 2018. PMID: 29625978 Free PMC article.
-
Anaerobic oxidation of methane: an "active" microbial process.Microbiologyopen. 2015 Feb;4(1):1-11. doi: 10.1002/mbo3.232. Epub 2014 Dec 22. Microbiologyopen. 2015. PMID: 25530008 Free PMC article. Review.
-
Comparative genomics reveals electron transfer and syntrophic mechanisms differentiating methanotrophic and methanogenic archaea.PLoS Biol. 2022 Jan 5;20(1):e3001508. doi: 10.1371/journal.pbio.3001508. eCollection 2022 Jan. PLoS Biol. 2022. PMID: 34986141 Free PMC article. Review.
Cited by
-
Deep-sea microbially influenced corrosion and biomineralization.Front Microbiol. 2025 Jul 17;16:1605909. doi: 10.3389/fmicb.2025.1605909. eCollection 2025. Front Microbiol. 2025. PMID: 40746314 Free PMC article. Review.
References
-
- Boetius A., et al. , A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 407, 623 (2000). - PubMed
-
- Reeburgh W. S., Oceanic methane biogeochemistry. Chem. Rev. 107, 486–513 (2007). - PubMed
-
- Wegener G., Laso-Pérez R., Orphan V. J., Boetius A., Anaerobic degradation of alkanes by marine archaea. Annu. Rev. Microbiol. 76, 553–577 (2022). - PubMed
-
- Luff R., Wallmann K., Fluid flow, methane fluxes, carbonate precipitation and biogeochemical turnover in gas hydrate-bearing sediments at Hydrate Ridge, Cascadia Margin: Numerical modeling and mass balances. Geochim. Cosmochim. Acta 67, 3403–3421 (2003).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

