Stereoelectroencephalography in the setting of a previously implanted responsive neural stimulation device: illustrative case
- PMID: 38079629
- PMCID: PMC10718281
- DOI: 10.3171/CASE23590
Stereoelectroencephalography in the setting of a previously implanted responsive neural stimulation device: illustrative case
Abstract
Background: Responsive neural stimulation (RNS) is a relatively novel procedure for drug-resistant epilepsy, which involves implantation of a device into the skull and brain. As more devices are implanted, there may be an increasing need to perform intracranial electrocorticography in implant patients with persistent seizures. Given the device location, imaging difficulties with implanted devices, and other technical hurdles, stereoelectroencephalography (SEEG) could be especially challenging. The authors describe the first reported SEEG investigation in a patient with an RNS device, highlighting the technical challenges and clinical data ascertained.
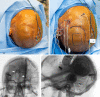
Observations: A 41-year-old male with drug-resistant epilepsy presented several years after a local surgeon had placed an RNS device with two electrodes in the bilateral parieto-occipital lobes. Because of inadequate seizure control, the patient was offered a repeat SEEG investigation to characterize his epilepsy better. Although more technically challenging than a traditional SEEG implantation, the SEEG investigation was successfully performed, which led to a confirmation of bilateral hippocampal seizure onset. The patient underwent repositioning of his RNS leads with a significant decrease in his seizure frequency.
Lessons: Concurrent implantation of SEEG electrodes in a functioning RNS device can be safely performed and can augment our understanding of a patient's seizures.
Keywords: epilepsy; functional neurosurgery; responsive neural stimulation; stereoelectroencephalography.
Conflict of interest statement
Figures


References
-
- Food and Drug Administration. Premarket Approval (PMA) Premarket Approval (PMA): Neuropace RNS System. Published September 25, 2023. Accessed September 26, 2023. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P100026.
-
- Geller EB, Skarpaas TL, Gross RE, et al. Brain-responsive neurostimulation in patients with medically intractable mesial temporal lobe epilepsy. Epilepsia. 2017;58(6):994–1004. - PubMed
-
- Jobst BC, Kapur R, Barkley GL, et al. Brain-responsive neurostimulation in patients with medically intractable seizures arising from eloquent and other neocortical areas. Epilepsia. 2017;58(6):1005–1014. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

