Replication-Competent Virus Detected in Blood of a Fatal COVID-19 Case
- PMID: 38079637
- PMCID: PMC10714275
- DOI: 10.7326/L23-0253
Replication-Competent Virus Detected in Blood of a Fatal COVID-19 Case
Conflict of interest statement
Figures
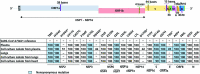
References
-
- Saá P, Fink RV, Bakkour S, et al; NHLBI Recipient Epidemiology and Donor Evaluation Study-IV-Pediatric (REDS-IV-P). Frequent detection but lack of infectivity of SARS-CoV-2 RNA in presymptomatic, infected blood donor plasma. J Clin Invest. 2022;132:e159876. [PMID: ] doi:10.1172/JCI159876 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
