In vitro dose-dependent effects of matrix metalloproteinases on ECM hydrogel biodegradation
- PMID: 38081445
- PMCID: PMC10775082
- DOI: 10.1016/j.actbio.2023.12.003
In vitro dose-dependent effects of matrix metalloproteinases on ECM hydrogel biodegradation
Abstract
Matrix metalloproteinases (MMPs) cause proteolysis of extracellular matrix (ECM) in tissues affected by stroke. However, little is known about how MMPs degrade ECM hydrogels implanted into stroke cavities to regenerate lost tissue. To establish a structure-function relationship between different doses of individual MMPs and isolate their effects in a controlled setting, an in vitro degradation assay quantified retained urinary bladder matrix (UBM) hydrogel mass as a measure of degradation across time. A rheological characterization indicated that lower ECM concentrations (<4 mg/mL) did not cure completely at 37 °C and had a high fraction of mobile proteins that were easily washed-out. Hydrolysis by dH2O caused a steady 2 % daily decrease in hydrogel mass over 14 days. An acceleration of degradation to 6 % occurred with phosphate buffered saline and artificial cerebrospinal fluid. MMPs induced a dose-dependent increase and within 14 days almost completely (>95 %) degraded the hydrogel. MMP-9 exerted the most significant biodegradation, compared to MMP-3 and -2. To model the in vivo exposure of hydrogel to MMPs, mixtures of MMP-2, -3, and -9, present in the cavity at 14-, 28-, or 90-days post-stroke, revealed that 14- and 28-days mixtures achieved an equivalent biodegradation, but a 90-days mixture exhibited a slower degradation. These results revealed that hydrolysis, in addition to proteolysis, exerts a major influence on the degradation of hydrogels. Understanding the mechanisms of ECM hydrogel biodegradation is essential to determine the therapeutic window for bioscaffold implantation after a stroke, and they are also key to determine optimal degradation kinetics to support tissue regeneration. STATEMENT OF SIGNIFICANCE: After implantation into a stroke cavity, extracellular matrix (ECM) hydrogel promotes tissue regeneration through the degradation of the bioscaffold. However, the process of degradation of an ECM hydrogel remains poorly understood. We here demonstrated in vitro under highly controlled conditions that hydrogel degradation is very dependent on its protein concentration. Lower protein concentration hydrogels were weaker in rheological measurements and particularly susceptible to hydrolysis. The proteolytic degradation of tissue ECM after a stroke is caused by matrix metalloproteinases (MMPs). A dose-dependent MMP-driven biodegradation of ECM hydrogel exceeded the effects of hydrolysis. These results highlight the importance of in vitro testing of putative causes of degradation to gain a better understanding of how these factors affect in vivo biodegradation.
Keywords: Biodegradation; Bioscaffold; Extracellular matrix; Hydrogel; Matrix metalloproteinase; Rheology.
Copyright © 2023 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Disclosures Dr. Badylak is the chief scientific officer and holds a vested interest in ECM-Therapeutics, a company that manufactures ECM-based hydrogels.
Figures




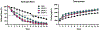

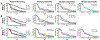
References
-
- Modo M, Ambrosio F, Friedlander RM, Badylak SF, Wechsler LR, Bioengineering solutions for neural repair and recovery in stroke, Curr Opin Neurol 26(6) (2013) 626–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

