Knockout of the inhibitory receptor TIGIT enhances the antitumor response of ex vivo expanded NK cells and prevents fratricide with therapeutic Fc-active TIGIT antibodies
- PMID: 38081778
- PMCID: PMC10729131
- DOI: 10.1136/jitc-2023-007502
Knockout of the inhibitory receptor TIGIT enhances the antitumor response of ex vivo expanded NK cells and prevents fratricide with therapeutic Fc-active TIGIT antibodies
Abstract
Background: Inhibitory receptor T-cell Immunoreceptor with Ig and ITIM domains (TIGIT) expressed by Natural Killer (NK) and T cells regulates cancer immunity and has been touted as the next frontier in the development of cancer immunotherapeutics. Although early results of anti-TIGIT and its combinations with antiprogrammed death-ligand 1 were highly exciting, results from an interim analysis of phase III trials are disappointing. With mixed results, there is a need to understand the effects of therapeutic anti-TIGIT on the TIGIT+ immune cells to support its clinical use. Most of the TIGIT antibodies in development have an Fc-active domain, which binds to Fc receptors on effector cells. In mouse models, Fc-active anti-TIGIT induced superior immunity, while Fc receptor engagement was required for its efficacy. NK-cell depletion compromised the antitumor immunity of anti-TIGIT indicating the essential role of NK cells in the efficacy of anti-TIGIT. Since NK cells express TIGIT and Fc-receptor CD16, Fc-active anti-TIGIT may deplete NK cells via fratricide, which has not been studied.
Methods: CRISPR-Cas9-based TIGIT knockout (KO) was performed in expanded NK cells. Phenotypic and transcriptomic properties of TIGIT KO and wild-type (WT) NK cells were compared with flow cytometry, CyTOF, and RNA sequencing. The effect of TIGIT KO on NK-cell cytotoxicity was determined by calcein-AM release and live cell imaging-based cytotoxicity assays. The metabolic properties of TIGIT KO and WT NK cells were compared with a Seahorse analyzer. The effect of the Fc-component of anti-TIGIT on NK-cell fratricide was determined by co-culturing WT and TIGIT KO NK cells with Fc-active and Fc-inactive anti-TIGIT.
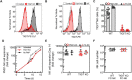
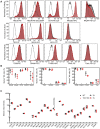
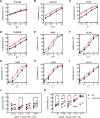
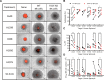
Results: TIGIT KO increased the cytotoxicity of NK cells against multiple cancer cell lines including spheroids. TIGIT KO NK cells upregulated mTOR complex 1 (mTORC1) signaling and had better metabolic fitness with an increased basal glycolytic rate when co-cultured with cancer cells compared with WT NK cells. Importantly, TIGIT KO prevented NK-cell fratricide when combined with Fc-active anti-TIGIT.
Conclusions: TIGIT KO in ex vivo expanded NK cells increased their cytotoxicity and metabolic fitness and prevented NK-cell fratricide when combined with Fc-active anti-TIGIT antibodies. These fratricide-resistant TIGIT KO NK cells have therapeutic potential alone or in combination with Fc-active anti-TIGIT antibodies to enhance their efficacy.
Keywords: cell engineering; immune checkpoint inhibitors; immunotherapy, adoptive.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: AJC: licensed IP to, consultancy and research support from Kiadis Pharma, a Sanofi company; JLO: licensed IP to, consultancy with Kiadis Pharma, a Sanofi company. MFH, TJC-P: licensed IP to Kiadis Pharma, a Sanofi company; DL: scientific advisory board of and consults for Avidicure; consultancy, licensing, and royalty fees from Kiadis Pharma, a Sanofi Company; IP interests related to NK-cell therapy.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
