ComplexEye: a multi-lens array microscope for high-throughput embedded immune cell migration analysis
- PMID: 38081825
- PMCID: PMC10713721
- DOI: 10.1038/s41467-023-43765-3
ComplexEye: a multi-lens array microscope for high-throughput embedded immune cell migration analysis
Abstract
Autonomous migration is essential for the function of immune cells such as neutrophils and plays an important role in numerous diseases. The ability to routinely measure or target it would offer a wealth of clinical applications. Video microscopy of live cells is ideal for migration analysis, but cannot be performed at sufficiently high-throughput (HT). Here we introduce ComplexEye, an array microscope with 16 independent aberration-corrected glass lenses spaced at the pitch of a 96-well plate to produce high-resolution movies of migrating cells. With the system, we enable HT migration analysis of immune cells in 96- and 384-well plates with very energy-efficient performance. We demonstrate that the system can measure multiple clinical samples simultaneously. Furthermore, we screen 1000 compounds and identify 17 modifiers of migration in human neutrophils in just 4 days, a task that requires 60-times longer with a conventional video microscope. ComplexEye thus opens the field of phenotypic HT migration screens and enables routine migration analysis for the clinical setting.
© 2023. The Author(s).
Conflict of interest statement
M.G., R.V., S.O. and A.G. have filed a patent application in Germany and worldwide (WO2018019406A3) for the imaging concept of multi-lens video microscopy as described in this work. All other authors declare no competing interests. A.-K.K., J.E.E. and B.K. are employees of the LDC GmbH and have no competing interests.
Figures


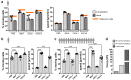
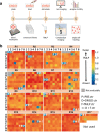

References
Publication types
MeSH terms
Grants and funding
- 2014-0050/Stiftung Mercator Schweiz (Mercator Foundation Switzerland)
- CRC TRR332 TP C6/Deutsche Forschungsgemeinschaft (German Research Foundation)
- KFO 337 "PhenoTime" TP7/Deutsche Forschungsgemeinschaft (German Research Foundation)
- FOR-2879 "Immunostroke" project 405358801/Deutsche Forschungsgemeinschaft (German Research Foundation)
- GU769/10-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
LinkOut - more resources
Full Text Sources

