Interleukin 31 receptor α promotes smooth muscle cell contraction and airway hyperresponsiveness in asthma
- PMID: 38081868
- PMCID: PMC10713652
- DOI: 10.1038/s41467-023-44040-1
Interleukin 31 receptor α promotes smooth muscle cell contraction and airway hyperresponsiveness in asthma
Abstract
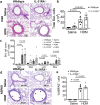
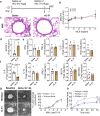
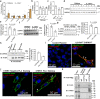
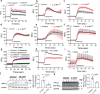
Asthma is a chronic inflammatory airway disease characterized by airway hyperresponsiveness (AHR), inflammation, and goblet cell hyperplasia. Multiple cytokines, including IFNγ, IL-4, and IL-13 are associated with asthma; however, the mechanisms underlying the effects of these cytokines remain unclear. Here, we report a significant increase in the expression of IL-31RA, but not its cognate ligand IL-31, in mouse models of allergic asthma. In support of this, IFNγ, IL-4, and IL-13 upregulated IL-31RA but not IL-31 in both human and mice primary airway smooth muscle cells (ASMC) isolated from the airways of murine and human lungs. Importantly, the loss of IL-31RA attenuated AHR but had no effect on inflammation and goblet cell hyperplasia in mice challenged with allergens or treated with IL-13 or IFNγ. We show that IL-31RA functions as a positive regulator of muscarinic acetylcholine receptor 3 expression, augmenting calcium levels and myosin light chain phosphorylation in human and murine ASMC. These findings identify a role for IL-31RA in AHR that is distinct from airway inflammation and goblet cell hyperplasia in asthma.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Update of
-
Interleukin 31 receptor alpha augments muscarinic acetylcholine receptor 3-driven calcium signaling and airway hyperresponsiveness in asthma.Res Sq [Preprint]. 2023 Feb 15:rs.3.rs-2564484. doi: 10.21203/rs.3.rs-2564484/v1. Res Sq. 2023. Update in: Nat Commun. 2023 Dec 11;14(1):8207. doi: 10.1038/s41467-023-44040-1. PMID: 36824812 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

