Energy-driven genome regulation by ATP-dependent chromatin remodellers
- PMID: 38081975
- PMCID: PMC12303036
- DOI: 10.1038/s41580-023-00683-y
Energy-driven genome regulation by ATP-dependent chromatin remodellers
Abstract
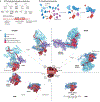
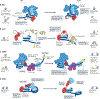
The packaging of DNA into chromatin in eukaryotes regulates gene transcription, DNA replication and DNA repair. ATP-dependent chromatin remodelling enzymes (re)arrange nucleosomes at the first level of chromatin organization. Their Snf2-type motor ATPases alter histone-DNA interactions through a common DNA translocation mechanism. Whether remodeller activities mainly catalyse nucleosome dynamics or accurately co-determine nucleosome organization remained unclear. In this Review, we discuss the emerging mechanisms of chromatin remodelling: dynamic remodeller architectures and their interactions, the inner workings of the ATPase cycle, allosteric regulation and pathological dysregulation. Recent mechanistic insights argue for a decisive role of remodellers in the energy-driven self-organization of chromatin, which enables both stability and plasticity of genome regulation - for example, during development and stress. Different remodellers, such as members of the SWI/SNF, ISWI, CHD and INO80 families, process (epi)genetic information through specific mechanisms into distinct functional outputs. Combinatorial assembly of remodellers and their interplay with histone modifications, histone variants, DNA sequence or DNA-bound transcription factors regulate nucleosome mobilization or eviction or histone exchange. Such input-output relationships determine specific nucleosome positions and compositions with distinct DNA accessibilities and mediate differential genome regulation. Finally, remodeller genes are often mutated in diseases characterized by genome dysregulation, notably in cancer, and we discuss their physiological relevance.
© 2023. Springer Nature Limited.
Conflict of interest statement
Conflict of interest
The authors declare no competing interests.
Figures


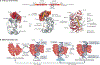



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

