An α-chain modification rivals the effect of fetal hemoglobin in retarding the rate of sickle cell fiber formation
- PMID: 38081985
- PMCID: PMC10713580
- DOI: 10.1038/s41598-023-48919-3
An α-chain modification rivals the effect of fetal hemoglobin in retarding the rate of sickle cell fiber formation
Abstract
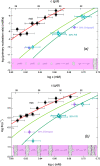
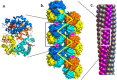
Adults with sickle cell disease bear a mutation in the β-globin gene, leading to the expression of sickle hemoglobin (HbS; α2βS2). Adults also possess the gene for γ-globin, which is a component of fetal hemoglobin (HbF, α2γ2); however, γ-chain expression normally ceases after birth. As HbF does not form the fibers that cause the disease, pharmacological and gene-modifying interventions have attempted to either reactivate expression of the γ chain or introduce a gene encoding a modified β chain having γ-like character. Here, we show that a single-site modification on the α chain, αPro114Arg, retards fiber formation as effectively as HbF. Because this addition to the repertoire of anti-sickling approaches acts independently of other modifications, it could be coupled with other therapies to significantly enhance their effectiveness.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

