Response to febuxostat according to clinical subtypes of hyperuricemia: a prospective cohort study in primary gout
- PMID: 38082308
- PMCID: PMC10712161
- DOI: 10.1186/s13075-023-03228-y
Response to febuxostat according to clinical subtypes of hyperuricemia: a prospective cohort study in primary gout
Abstract
Background: While xanthine oxidase inhibitors target uric acid production, renal urate underexcretion is the predominant subtypes in gout. This study was to compare treatment response to the XOI febuxostat in a gout cohort according to clinical subtypes of hyperuricemia.
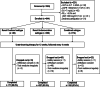
Methods: A prospective cohort study was conducted to compare the efficacy and safety of febuxostat (initially 20 mg daily, escalating to 40 mg daily if not at target) in 644 gout patients with the three major clinical subtypes for 12 weeks. Hyperuricemia was defined as the renal overload subtype, the renal underexcretion subtype, or the combined subtype based on UUE > or ≤ 600 mg/d/1.73 m2 and FEUA < or ≥ 5.5%. The primary endpoint was the rate of achieving serum urate (SU) < 6 mg/dL at week 12.
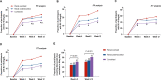
Results: Fewer participants with combined subtype achieved the SU target, 45.5% compared with 64.8% with overload subtype (P = 0.007), and 56.6% with underexcretion subtype (P = 0.022). More participants with combined subtype (82%) had febuxostat escalated to 40 mg than those with overload (62%, P = 0.001) or underexcretion subtype (68%, P = 0.001). In all participants, combined subtype hyperuricemia (OR = 0.64, 95%CI 0.41-0.99, P = 0.048) and baseline SU (OR = 0.74, 95%CI 0.62-0.89, P = 0.001) were independently associated with lower rates of achieving SU target.
Conclusions: People with combined subtype have a lower response to febuxostat, compared to those with either overload or underexcretion subtype. Assessment of hyperuricemia subtype may provide useful clinical data in predicting febuxostat response.
Keywords: Clinical subtypes of hyperuricemia; Febuxostat; Gout.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Superiority of Low-Dose Benzbromarone to Low-Dose Febuxostat in a Prospective, Randomized Comparative Effectiveness Trial in Gout Patients With Renal Uric Acid Underexcretion.Arthritis Rheumatol. 2022 Dec;74(12):2015-2023. doi: 10.1002/art.42266. Epub 2022 Nov 11. Arthritis Rheumatol. 2022. PMID: 35795968 Free PMC article. Clinical Trial.
-
Superiority of Low-Dose Benzbromarone Add-On to Low-Dose Febuxostat Compared With Febuxostat Monotherapy in Gout With Combined-Type Hyperuricemia.Arthritis Care Res (Hoboken). 2024 May;76(5):703-711. doi: 10.1002/acr.25283. Epub 2024 Feb 20. Arthritis Care Res (Hoboken). 2024. PMID: 38130040 Free PMC article. Clinical Trial.
-
Febuxostat: a selective xanthine-oxidase/xanthine-dehydrogenase inhibitor for the management of hyperuricemia in adults with gout.Clin Ther. 2009 Nov;31(11):2503-18. doi: 10.1016/j.clinthera.2009.11.033. Clin Ther. 2009. PMID: 20109996 Review.
-
Comparison of efficacy and safety of urate-lowering therapies for hyperuricemic patients with gout: a meta-analysis of randomized, controlled trials.Clin Rheumatol. 2021 Feb;40(2):683-692. doi: 10.1007/s10067-020-05272-4. Epub 2020 Jul 11. Clin Rheumatol. 2021. PMID: 32654080
-
Management of hyperuricemia in gout: focus on febuxostat.Clin Interv Aging. 2010 Feb 2;5:7-18. doi: 10.2147/cia.s5476. Clin Interv Aging. 2010. PMID: 20169038 Free PMC article. Review.
Cited by
-
Predictors of Inadequate Serum Urate Response to Low-Dose Febuxostat in Male Patients with Gout.J Inflamm Res. 2024 Apr 30;17:2657-2668. doi: 10.2147/JIR.S458250. eCollection 2024. J Inflamm Res. 2024. PMID: 38707960 Free PMC article.
References
-
- C.M B, R.L W. Kelley and Firestein’s Textbook of Rheumatology (the 10th edition) 1633 (Elsevier, 2017).
Publication types
MeSH terms
Substances
Grants and funding
- 81871288, 81900636/National Natural Science Foundation of China
- 81871288, 81900636/National Natural Science Foundation of China
- 2022YFC2503300/National Key Research and Development Program of China
- 82220108015/Projects of International Cooperation and Exchanges NSFC
- 2021CXGC011103/Shandong Provincial Key Research and Development Plan Major Scientific and Technological Innovation Project
LinkOut - more resources
Full Text Sources
Medical

