Bufalin targeting CAMKK2 inhibits the occurrence and development of intrahepatic cholangiocarcinoma through Wnt/β-catenin signal pathway
- PMID: 38082327
- PMCID: PMC10714474
- DOI: 10.1186/s12967-023-04613-6
Bufalin targeting CAMKK2 inhibits the occurrence and development of intrahepatic cholangiocarcinoma through Wnt/β-catenin signal pathway
Abstract
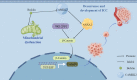
Background: Intrahepatic cholangiocarcinoma (ICC) accounts for about 15% of primary liver cancer, and the incidence rate has been rising in recent years. Surgical resection is the best treatment for ICC, but the 5-year survival rate is less than 30%. ICC signature genes are crucial for the early diagnosis of ICC, so it is especially important to find its signature genes and therapeutic drug. Here, we studied that bufalin targeting CAMKK2 promotes mitochondrial dysfunction and inhibits the occurrence and metastasis of intrahepatic cholangiocarcinoma through Wnt/β-catenin signal pathway.
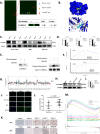
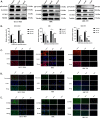
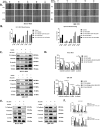
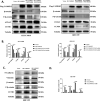
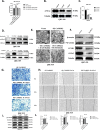
Methods: IC50 of bufalin in ICC cells was determined by CCK8 and invasive and migratory abilities were verified by wound healing, cell cloning, transwell and Western blot. IF and IHC verified the expression of CAMKK2 between ICC patients and normal subjects. BLI and pull-down demonstrated the binding ability of bufalin and CAMKK2. Bioinformatics predicted whether CAMKK2 was related to the Wnt/β-catenin pathway. SKL2001, an activator of β-catenin, verified whether bufalin acted through this pathway. In vitro and in vivo experiments verified whether overexpression of CAMKK2 affects the proliferative and migratory effects of ICC. Transmission electron microscopy verified mitochondrial integrity. Associated Ca2+ levels verified the biological effects of ANXA2 on ICC.
Results: It was found that bufalin inhibited the proliferation and migration of ICC, and CAMKK2 was highly expressed in ICC, and its high expression was positively correlated with poor prognosis.CAMKK2 is a direct target of bufalin, and is associated with the Wnt/β-catenin signaling pathway, which was dose-dependently decreased after bufalin treatment. In vitro and in vivo experiments verified that CAMKK2 overexpression promoted ICC proliferation and migration, and bufalin reversed this effect. CAMKK2 was associated with Ca2+, and changes in Ca2+ content induced changes in the protein content of ANXA2, which was dose-dependently decreasing in cytoplasmic ANXA2 and dose-dependently increasing in mitochondrial ANXA2 after bufalin treatment. In CAMKK2 overexpressing cells, ANXA2 was knocked down, and we found that reversal of CAMKK2 overexpression-induced enhancement of ICC proliferation and migration after siANXA2.
Conclusions: Our results suggest that bufalin targeting CAMKK2 promotes mitochondrial dysfunction and inhibits the proliferation and migration of intrahepatic cholangiocarcinoma through Wnt/β-catenin signal pathway. Thus, bufalin, as a drug, may also be used for cancer therapy in ICC in the future.
Keywords: ANXA2; Bufalin; CAMKK2; Ca2+; Intrahepatic cholangiocarcinoma; Migration; Mitochondrial dysfunction; Proliferation.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Fattahi M, Shahrabi S, Saadatpour F, Rezaee D, Beyglu Z, Delavari S, Amrolahi A, Ahmadi S, Bagheri-Mohammadi S, Noori E, Majidpoor J, Nouri S, Aghaei-Zarch SM, Falahi S, Najafi S, Le BN. microRNA-382 as a tumor suppressor? Roles in tumorigenesis and clinical significance. Int J Biol Macromol. 2023;250:125863. doi: 10.1016/j.ijbiomac.2023.125863. - DOI - PubMed
-
- Faramin Lashkarian M, Hashemipour N, Niaraki N, Soghala S, Moradi A, Sarhangi S, Hatami M, Aghaei-Zarch F, Khosravifar M, Mohammadzadeh A, Najafi S, Majidpoor J, Farnia P, Aghaei-Zarch SM. MicroRNA-122 in human cancers: from mechanistic to clinical perspectives. Cancer Cell Int. 2023;23(1):29. doi: 10.1186/s12935-023-02868-z. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

