A multicomponent intervention program to Prevent and Reduce AgItation and phySical rEstraint use in the ICU (PRAISE): study protocol for a multicenter, stepped-wedge, cluster randomized controlled trial
- PMID: 38082351
- PMCID: PMC10712112
- DOI: 10.1186/s13063-023-07807-x
A multicomponent intervention program to Prevent and Reduce AgItation and phySical rEstraint use in the ICU (PRAISE): study protocol for a multicenter, stepped-wedge, cluster randomized controlled trial
Abstract
Background: Physical restraints remain to be commonly used in agitated intensive care unit (ICU) patients worldwide, despite a lack of evidence on efficacy and safety and reports of detrimental short and long-term consequences, such as prolonged delirium and a longer ICU length of stay. Physical restraint minimization approaches have focused mainly on educational strategies and other non-pharmacological interventions. Combining these interventions with goal-directed light sedation therapy if needed may play an important contributory role in further reducing the use of physical restraints. The aim of the study is to determine the effectiveness of a multicomponent intervention (MCI) program, combining person-centered non-pharmacological interventions with goal-directed light sedation, compared to physical restraints.
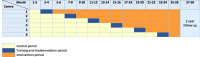
Methods: A multicenter stepped-wedge cluster randomized controlled trial will be conducted in six Dutch ICUs. A power calculation based total of 480 (expected to become) agitated adult patients will be included in 26 months with a subsequent 2-year follow-up. Patients included in the control period will receive standard care with the current agitation management protocol including physical restraints. Patients included in the intervention period will be treated with the MCI program, consisting of four components, without physical restraints: education of ICU professionals, identification of patients at risk for agitation, formulation of a multidisciplinary person-centered care plan including non-pharmacological and medical interventions, and protocolized goal-directed light sedation using dexmedetomidine. Primary outcome is the number of days alive and outside of the ICU within 28 days after ICU admission. Secondary outcomes include length of hospital stay; 3-, 12-, and 24-month post-ICU quality of life; physical (fatigue, frailty, new physical problems), mental (anxiety, depression, and post-traumatic stress disorder), and cognitive health; and 1-year cost-effectiveness. A process evaluation will be conducted.
Discussion: This will be the first multicenter randomized controlled trial determining the effect of a combination of non-pharmacological interventions and light sedation using dexmedetomidine compared to physical restraints in agitated ICU patients. The results of this study, including long-term patient-centered outcomes, will provide relevant insights to aid ICU professionals in the management of agitated patients.
Trial registration: NCT05783505, registration date 23 March 2023.
Keywords: Agitation; Dexmedetomidine; ICU; Non-pharmacological interventions; Patient-reported outcome measures; Physical restraints.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJC, Pandharipande PP, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825–e873. doi: 10.1097/CCM.0000000000003299. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical