Effects of Empagliflozin on Fluid Overload, Weight, and Blood Pressure in CKD
- PMID: 38082486
- PMCID: PMC7615589
- DOI: 10.1681/ASN.0000000000000271
Effects of Empagliflozin on Fluid Overload, Weight, and Blood Pressure in CKD
Abstract
Significance statement: SGLT2 inhibitors reduce risk of kidney progression, AKI, and cardiovascular disease, but the mechanisms of benefit are incompletely understood. Bioimpedance spectroscopy can estimate body water and fat mass. One quarter of the EMPA-KIDNEY bioimpedance substudy CKD population had clinically significant levels of bioimpedance-derived "Fluid Overload" at recruitment. Empagliflozin induced a prompt and sustained reduction in "Fluid Overload," irrespective of sex, diabetes, and baseline N-terminal pro B-type natriuretic peptide or eGFR. No significant effect on bioimpedance-derived fat mass was observed. The effects of SGLT2 inhibitors on body water may be one of the contributing mechanisms by which they mediate effects on cardiovascular risk.
Background: CKD is associated with fluid excess that can be estimated by bioimpedance spectroscopy. We aimed to assess effects of sodium glucose co-transporter 2 inhibition on bioimpedance-derived "Fluid Overload" and adiposity in a CKD population.
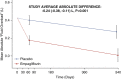
Methods: EMPA-KIDNEY was a double-blind placebo-controlled trial of empagliflozin 10 mg once daily in patients with CKD at risk of progression. In a substudy, bioimpedance measurements were added to the main trial procedures at randomization and at 2- and 18-month follow-up visits. The substudy's primary outcome was the study-average difference in absolute "Fluid Overload" (an estimate of excess extracellular water) analyzed using a mixed model repeated measures approach.
Results: The 660 substudy participants were broadly representative of the 6609-participant trial population. Substudy mean baseline absolute "Fluid Overload" was 0.4±1.7 L. Compared with placebo, the overall mean absolute "Fluid Overload" difference among those allocated empagliflozin was -0.24 L (95% confidence interval [CI], -0.38 to -0.11), with similar sized differences at 2 and 18 months, and in prespecified subgroups. Total body water differences comprised between-group differences in extracellular water of -0.49 L (95% CI, -0.69 to -0.30, including the -0.24 L "Fluid Overload" difference) and a -0.30 L (95% CI, -0.57 to -0.03) difference in intracellular water. There was no significant effect of empagliflozin on bioimpedance-derived adipose tissue mass (-0.28 kg [95% CI, -1.41 to 0.85]). The between-group difference in weight was -0.7 kg (95% CI, -1.3 to -0.1).
Conclusions: In a broad range of patients with CKD, empagliflozin resulted in a sustained reduction in a bioimpedance-derived estimate of fluid overload, with no statistically significant effect on fat mass.
Trial registration: Clinicaltrials.gov: NCT03594110 ; EuDRACT: 2017-002971-24 ( https://eudract.ema.europa.eu/ ).
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Society of Nephrology.
Conflict of interest statement
The EMPA-KIDNEY bioimpedance substudy was initiated, designed, conducted, analyzed, and reported by the University of Oxford with a steering committee of experts. This paper has not been published previously in whole or part. The Clinical Trial Service Unit and Epidemiological Studies Unit (Oxford, UK) have a staff policy of not accepting honorarium or other payments from the pharmaceutical industry, except for the reimbursement of costs to participate in scientific meetings (see
Figures




References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

