Neurotropic virus infection and neurodegenerative diseases: Potential roles of autophagy pathway
- PMID: 38082503
- PMCID: PMC11163195
- DOI: 10.1111/cns.14548
Neurotropic virus infection and neurodegenerative diseases: Potential roles of autophagy pathway
Abstract
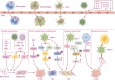
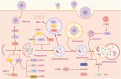
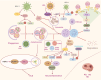
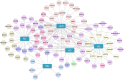
Neurodegenerative diseases (NDs) constitute a group of disorders characterized by the progressive deterioration of nervous system functionality. Currently, the precise etiological factors responsible for NDs remain incompletely elucidated, although it is probable that a combination of aging, genetic predisposition, and environmental stressors participate in this process. Accumulating evidence indicates that viral infections, especially neurotropic viruses, can contribute to the onset and progression of NDs. In this review, emerging evidence supporting the association between viral infection and NDs is summarized, and how the autophagy pathway mediated by viral infection can cause pathological aggregation of cellular proteins associated with various NDs is discussed. Furthermore, autophagy-related genes (ARGs) involved in Herpes simplex virus (HSV-1) infection and NDs are analyzed, and whether these genes could link HSV-1 infection to NDs is discussed. Elucidating the mechanisms underlying NDs is critical for developing targeted therapeutic approaches that prevent the onset and slow the progression of NDs.
Keywords: Alzheimer's disease; Parkinson's disease; autophagy; neurodegenerative diseases; neurotropic virus.
© 2023 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
All authors declare no financial or non‐financial competing interests.
Figures

Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Stress-induced Cdk5 activity enhances cytoprotective basal autophagy in Drosophila melanogaster by phosphorylating acinus at serine437.Elife. 2017 Dec 11;6:e30760. doi: 10.7554/eLife.30760. Elife. 2017. PMID: 29227247 Free PMC article.
-
Anti-herpes simplex virus type 1 activity of the Rohdea chinensis (Baker) N. Tanaka aqueous extracts.J Ethnopharmacol. 2025 Jun 26;350:120024. doi: 10.1016/j.jep.2025.120024. Epub 2025 May 22. J Ethnopharmacol. 2025. PMID: 40412773
-
Viruses and the Brain-A Relationship Prone to Trouble.Viruses. 2025 Jan 31;17(2):203. doi: 10.3390/v17020203. Viruses. 2025. PMID: 40006958 Free PMC article. Review.
-
Comprehensive Exploration of the Neuroprotective Mechanisms of Ginkgo biloba Leaves in Treating Neurological Disorders.Am J Chin Med. 2024;52(4):1053-1086. doi: 10.1142/S0192415X24500435. Epub 2024 Jun 21. Am J Chin Med. 2024. PMID: 38904550
Cited by
-
Nanomaterial-based approaches to neurotoxin neutralization in neurodegenerative diseases.Nanomedicine (Lond). 2025 May;20(9):1015-1027. doi: 10.1080/17435889.2025.2487409. Epub 2025 Apr 3. Nanomedicine (Lond). 2025. PMID: 40181662 Review.
-
Underestimated virus impaired cognition-more evidence and more work to do.Front Immunol. 2025 May 12;16:1550179. doi: 10.3389/fimmu.2025.1550179. eCollection 2025. Front Immunol. 2025. PMID: 40421014 Free PMC article. Review.
-
Infections in the Etiology of Parkinson's Disease and Synucleinopathies: A Renewed Perspective, Mechanistic Insights, and Therapeutic Implications.J Parkinsons Dis. 2024;14(7):1301-1329. doi: 10.3233/JPD-240195. J Parkinsons Dis. 2024. PMID: 39331109 Free PMC article. Review.
-
Preparation, and ex vivo and in vivo Characterization of Favipiravir-Loaded Aspasomes and Niosomes for Nose-to-Brain Administration.Int J Nanomedicine. 2025 May 22;20:6489-6514. doi: 10.2147/IJN.S518486. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40420912 Free PMC article.
-
Reversing Epigenetic Dysregulation in Neurodegenerative Diseases: Mechanistic and Therapeutic Considerations.Int J Mol Sci. 2025 May 21;26(10):4929. doi: 10.3390/ijms26104929. Int J Mol Sci. 2025. PMID: 40430067 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

