Regulating Chondro-Bone Metabolism for Treatment of Osteoarthritis via High-Permeability Micro/Nano Hydrogel Microspheres
- PMID: 38084002
- PMCID: PMC10837371
- DOI: 10.1002/advs.202305023
Regulating Chondro-Bone Metabolism for Treatment of Osteoarthritis via High-Permeability Micro/Nano Hydrogel Microspheres
Abstract
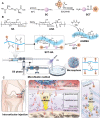
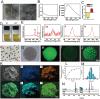
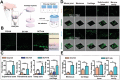
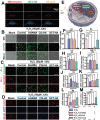
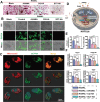
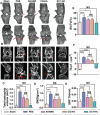
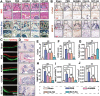
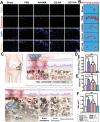
Destruction of cartilage due to the abnormal remodeling of subchondral bone (SB) leads to osteoarthritis (OA), and restoring chondro-bone metabolic homeostasis is the key to the treatment of OA. However, traditional intra-articular injections for the treatment of OA cannot directly break through the cartilage barrier to reach SB. In this study, the hydrothermal method is used to synthesize ultra-small size (≈5 nm) selenium-doped carbon quantum dots (Se-CQDs, SC), which conjugated with triphenylphosphine (TPP) to create TPP-Se-CQDs (SCT). Further, SCT is dynamically complexed with hyaluronic acid modified with aldehyde and methacrylic anhydride (AHAMA) to construct highly permeable micro/nano hydrogel microspheres (SCT@AHAMA) for restoring chondro-bone metabolic homeostasis. In vitro experiments confirmed that the selenium atoms scavenged reactive oxygen species (ROS) from the mitochondria of mononuclear macrophages, inhibited osteoclast differentiation and function, and suppressed early chondrocyte apoptosis to maintain a balance between cartilage matrix synthesis and catabolism. In vivo experiments further demonstrated that the delivery system inhibited osteoclastogenesis and H-vessel invasion, thereby regulating the initiation and process of abnormal bone remodeling and inhibiting cartilage degeneration in SB. In conclusion, the micro/nano hydrogel microspheres based on ultra-small quantum dots facilitate the efficient penetration of articular SB and regulate chondro-bone metabolism for OA treatment.
Keywords: high-permeability; hydrogel microsphere; osteoarthritis; subchondral bone.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Cascade targeting selenium nanoparticles-loaded hydrogel microspheres for multifaceted antioxidant defense in osteoarthritis.Biomaterials. 2025 Jul;318:123195. doi: 10.1016/j.biomaterials.2025.123195. Epub 2025 Feb 15. Biomaterials. 2025. PMID: 39965424
-
In Situ Remodeling of Efferocytosis via Lesion-Localized Microspheres to Reverse Cartilage Senescence.Adv Sci (Weinh). 2024 May;11(19):e2400345. doi: 10.1002/advs.202400345. Epub 2024 Mar 13. Adv Sci (Weinh). 2024. PMID: 38477444 Free PMC article.
-
Nanoarchitectonics of Cartilage-Targeting Hydrogel Microspheres with Reactive Oxygen Species Responsiveness for the Repair of Osteoarthritis.ACS Appl Mater Interfaces. 2022 Sep 14;14(36):40711-40723. doi: 10.1021/acsami.2c12703. Epub 2022 Sep 5. ACS Appl Mater Interfaces. 2022. PMID: 36063108
-
Targeting TGFβ signaling in subchondral bone and articular cartilage homeostasis.Trends Pharmacol Sci. 2014 May;35(5):227-36. doi: 10.1016/j.tips.2014.03.005. Epub 2014 Apr 15. Trends Pharmacol Sci. 2014. PMID: 24745631 Free PMC article. Review.
-
Chondrocyte Apoptosis in the Pathogenesis of Osteoarthritis.Int J Mol Sci. 2015 Oct 30;16(11):26035-54. doi: 10.3390/ijms161125943. Int J Mol Sci. 2015. PMID: 26528972 Free PMC article. Review.
Cited by
-
Functionalized Periosteum-Derived Microsphere-Hydrogel with Sequential Release of E7 Short Peptide/miR217 for Large Bone Defect Repairing.Biomater Res. 2025 Jan 7;29:0127. doi: 10.34133/bmr.0127. eCollection 2025. Biomater Res. 2025. PMID: 39780960 Free PMC article.
-
Modulation of the gut-bone axis: Lacticaseibacillus paracasei LC86 improves bone health via anti-inflammatory metabolic pathways in zebrafish models of osteoporosis and cartilage damage.Front Immunol. 2025 Apr 16;16:1493560. doi: 10.3389/fimmu.2025.1493560. eCollection 2025. Front Immunol. 2025. PMID: 40308595 Free PMC article.
-
Prevotella copri-mediated caffeine metabolism involves ferroptosis of osteoblasts in osteoarthritis.Microbiol Spectr. 2025 Jun 3;13(6):e0157524. doi: 10.1128/spectrum.01575-24. Epub 2025 Apr 24. Microbiol Spectr. 2025. PMID: 40272163 Free PMC article.
-
Cartilage-Penetrating Framework Nucleic Acid Nanoparticles Ameliorate Osteoarthritis by Promoting Drug Delivery and Chondrocyte Uptake.Adv Sci (Weinh). 2025 Jul;12(26):e2502661. doi: 10.1002/advs.202502661. Epub 2025 Apr 7. Adv Sci (Weinh). 2025. PMID: 40192172 Free PMC article.
-
Stem cell recruitment polypeptide hydrogel microcarriers with exosome delivery for osteoarthritis treatment.J Nanobiotechnology. 2024 Aug 27;22(1):512. doi: 10.1186/s12951-024-02765-1. J Nanobiotechnology. 2024. PMID: 39192268 Free PMC article.
References
-
- Liu Y., Xie H.‐Q., Shen B., Rheumatology 2023, 62, 1436. - PubMed
-
- Karsdal M. A., Leeming D. J., Dam E. B., Henriksen K., Alexandersen P., Pastoureau P., Altman R. D., Christiansen C., Osteoarthr. Cartil. 2008, 16, 638. - PubMed
-
- Burr D. B., Gallant M. A., Nat. Rev. Rheumatol. 2012, 8, 665. - PubMed
-
- Didomenico C. D., Lintz M., Bonassar L. J., Nat. Rev. Rheumatol. 2018, 14, 393. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
