Indole-3-Aldehyde Reduces Inflammatory Responses and Restores Intestinal Epithelial Barrier Function Partially via Aryl Hydrocarbon Receptor (AhR) in Experimental Colitis Models
- PMID: 38084103
- PMCID: PMC10710743
- DOI: 10.2147/JIR.S432747
Indole-3-Aldehyde Reduces Inflammatory Responses and Restores Intestinal Epithelial Barrier Function Partially via Aryl Hydrocarbon Receptor (AhR) in Experimental Colitis Models
Abstract
Purpose: Indole-3-aldehyde (IAld) has been shown to improve intestinal epithelial barrier (IEB) function through the aryl hydrocarbon receptor (AhR) in murine colitis models. However, the impact of IAld on intestinal tissue inflammation remains unexplored. This study aimed to investigate the effects of IAld on the inflammatory responses of the gut both in vivo and in vitro and probe the mechanisms by which IAld attenuates colitis.
Methods: The effects of IAld on phenotypic changes, pro-inflammatory cytokines, IEB functions and the faecal bacterial composition in mice with dextran sulfate sodium salt (DSS)-induced colitis were assessed. Macrophage cells and intestinal epithelial cells were stimulated with lipopolysaccharide (LPS), and the effects of IAld on the inflammatory responses and IBE functions were measured.
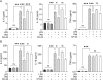
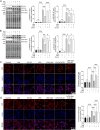
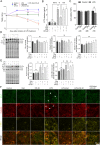
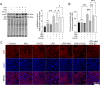
Results: IAld reduced IL-6, IL-1β and TNF-α protein levels in both colonic tissues from the mice with colitis and LPS-stimulated macrophage cells. The IAld-mediated reduction of IL-6 but not IL-1β and TNF-α was through AhR activation. Furthermore, nuclear factor-κB pathway was found to be inhibited by IAld treatment via AhR activation both in vivo and in vitro. Gut permeability was significantly improved by IAld in both DSS-treated mice and LPS-stimulated Caco-2 cells. This observation is consistent with downregulation of phosphorylated myosin light chain through AhR activation. IAld did not appear to have an effect on the bacterial composition in mice with colitis despite the reduced colonic inflammatory responses.
Conclusion: IAld improved DSS-induced colitis by inhibiting the inflammatory responses and restoring IEB function, partially via AhR activation. This work provided insight into the function of IAld in modulating gut inflammation.
Keywords: indole-3-aldehyde aryl hydrocarbon receptor intestinal inflammation.
© 2023 Wang et al.
Conflict of interest statement
The authors report no conflicts of interest in this work. Jia Li declared consultancy work for the University of Cardiff, the work is not relevant to the submitted manuscript.
Figures

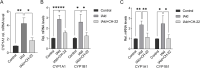
Similar articles
-
Indole-3-Carboxaldehyde Alleviates LPS-Induced Intestinal Inflammation by Inhibiting ROS Production and NLRP3 Inflammasome Activation.Antioxidants (Basel). 2024 Sep 13;13(9):1107. doi: 10.3390/antiox13091107. Antioxidants (Basel). 2024. PMID: 39334766 Free PMC article.
-
Gut microbiota-derived tryptophan metabolite indole-3-carboxaldehyde enhances intestinal barrier function via AhR/AMPK signaling activation.Anim Biosci. 2025 Jul 11. doi: 10.5713/ab.25.0225. Online ahead of print. Anim Biosci. 2025. PMID: 40665732
-
The Role of Lactiplantibacillus plantarum CGMCC9513 in Alleviating Colitis by Synergistic Enhancement of the Intestinal Barrier Through Modulating Gut Microbiota and Activating the Aryl Hydrocarbon Receptor.Probiotics Antimicrob Proteins. 2025 Apr 29. doi: 10.1007/s12602-025-10551-0. Online ahead of print. Probiotics Antimicrob Proteins. 2025. PMID: 40301232
-
The Aryl Hydrocarbon Receptor (AHR) as a Potential Target for the Control of Intestinal Inflammation: Insights from an Immune and Bacteria Sensor Receptor.Clin Rev Allergy Immunol. 2020 Dec;59(3):382-390. doi: 10.1007/s12016-020-08789-3. Clin Rev Allergy Immunol. 2020. PMID: 32279195 Review.
-
Therapeutic Potential of Nutritional Aryl Hydrocarbon Receptor Ligands in Gut-Related Inflammation and Diseases.Biomedicines. 2024 Dec 20;12(12):2912. doi: 10.3390/biomedicines12122912. Biomedicines. 2024. PMID: 39767818 Free PMC article. Review.
Cited by
-
Vulnerability of Antioxidant Drug Therapies on Targeting the Nrf2-Trp53-Jdp2 Axis in Controlling Tumorigenesis.Cells. 2024 Oct 3;13(19):1648. doi: 10.3390/cells13191648. Cells. 2024. PMID: 39404411 Free PMC article. Review.
-
Exploring the biological impact of bacteria-derived indole compounds on human cell health: Cytotoxicity and cell proliferation across six cell lines.Toxicol Rep. 2024 Dec 24;14:101883. doi: 10.1016/j.toxrep.2024.101883. eCollection 2025 Jun. Toxicol Rep. 2024. PMID: 39844884 Free PMC article.
-
Gut microbiome and metabolome interactions in Crohn's disease: mechanistic insights into exclusive enteral nutrition-induced remission.Front Microbiol. 2025 Jul 11;16:1616122. doi: 10.3389/fmicb.2025.1616122. eCollection 2025. Front Microbiol. 2025. PMID: 40718814 Free PMC article.
-
Gut Microbiome-Related Anti-Inflammatory Effects of Aryl Hydrocarbon Receptor Activation on Inflammatory Bowel Disease.Int J Mol Sci. 2024 Mar 16;25(6):3372. doi: 10.3390/ijms25063372. Int J Mol Sci. 2024. PMID: 38542367 Free PMC article. Review.
-
Indole-3-Carboxaldehyde Alleviates LPS-Induced Intestinal Inflammation by Inhibiting ROS Production and NLRP3 Inflammasome Activation.Antioxidants (Basel). 2024 Sep 13;13(9):1107. doi: 10.3390/antiox13091107. Antioxidants (Basel). 2024. PMID: 39334766 Free PMC article.
References
LinkOut - more resources
Full Text Sources

