Autonomic Dysreflexia in Spinal Cord Injury: Mechanisms and Prospective Therapeutic Targets
- PMID: 38084412
- PMCID: PMC11166887
- DOI: 10.1177/10738584231217455
Autonomic Dysreflexia in Spinal Cord Injury: Mechanisms and Prospective Therapeutic Targets
Abstract
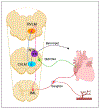
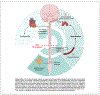
High-level spinal cord injury (SCI) often results in cardiovascular dysfunction, especially the development of autonomic dysreflexia. This disorder, characterized as an episode of hypertension accompanied by bradycardia in response to visceral or somatic stimuli, causes substantial discomfort and potentially life-threatening symptoms. The neural mechanisms underlying this dysautonomia include a loss of supraspinal control to spinal sympathetic neurons, maladaptive plasticity of sensory inputs and propriospinal interneurons, and excessive discharge of sympathetic preganglionic neurons. While neural control of cardiovascular function is largely disrupted after SCI, the renin-angiotensin system (RAS), which mediates blood pressure through hormonal mechanisms, is up-regulated after injury. Whether the RAS engages in autonomic dysreflexia, however, is still controversial. Regarding therapeutics, transplantation of embryonic presympathetic neurons, collected from the brainstem or more specific raphe regions, into the injured spinal cord may reestablish supraspinal regulation of sympathetic activity for cardiovascular improvement. This treatment reduces the occurrence of spontaneous autonomic dysreflexia and the severity of artificially triggered dysreflexic responses in rodent SCI models. Though transplanting early-stage neurons improves neural regulation of blood pressure, hormonal regulation remains high and baroreflex dysfunction persists. Therefore, cell transplantation combined with selected RAS inhibition may enhance neuroendocrine homeostasis for cardiovascular recovery after SCI.
Keywords: blood pressure; neural relay; renin-angiotensin system; serotonergic modulation; sympathetic regulation.
Conflict of interest statement
Declaration of Conflicting InterestsThe authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


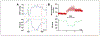
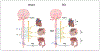


References
-
- Anderson KD. 2004. Targeting recovery: priorities of the spinal cord–injured population. J Neurotrauma 21(10):1371–83. - PubMed
-
- Averill DB, Diz DI. 2000. Angiotensin peptides and baroreflex control of sympathetic outflow: pathways and mechanisms of the medulla oblongata. Brain Res Bull 51(2):119–28. - PubMed
-
- Bollag WB. 2014. Regulation of aldosterone synthesis and secretion. Compr Physiol 4(3):1017–55. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

