Oral Carbon Monoxide Enhances Autophagy Modulation in Prostate, Pancreatic, and Lung Cancers
- PMID: 38084435
- PMCID: PMC10916612
- DOI: 10.1002/advs.202308346
Oral Carbon Monoxide Enhances Autophagy Modulation in Prostate, Pancreatic, and Lung Cancers
Abstract
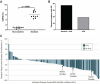
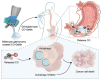
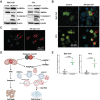
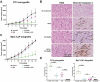
Modulation of autophagy, specifically its inhibition, stands to transform the capacity to effectively treat a broad range of cancers. However, the clinical efficacy of autophagy inhibitors has been inconsistent. To delineate clinical and epidemiological features associated with autophagy inhibition and a positive oncological clinical response, a retrospective analysis of patients is conducted treated with hydroxychloroquine, a known autophagy inhibitor. A direct correlation between smoking status and inhibition of autophagy with hydroxychloroquine is identified. Recognizing that smoking is associated with elevated circulating levels of carbon monoxide (CO), it is hypothesized that supplemental CO can amplify autophagy inhibition. A novel, gas-entrapping material containing CO in a pre-clinical model is applied and demonstrated that CO can dramatically increase the cytotoxicity of autophagy inhibitors and significantly inhibit the growth of tumors when used in combination. These data support the notion that safe, therapeutic levels of CO can markedly enhance the efficacy of autophagy inhibitors, opening a promising new frontier in the quest to improve cancer therapies.
Keywords: CO biofoams; autophagy modulation; combination therapies; smoking.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
HB, DG, LEO, GT, and JDB are co‐inventors on a patent application (WO2022055991A1) submitted by Brigham and Women's Hospital, MIT, and BIDMC that covers therapeutic carbon monoxide formulations. Complete details of all relationships for profit and non for profit for GT can be found at
Figures


References
-
- Mizushima N., Nat. Cell Biol. 2018, 20, 521. - PubMed
-
- a) Amaravadi R. K., Autophagy 2022, 18, 1470; - PMC - PubMed
- b) Arora S. P., Tenner L., Sarantopoulos J., Morris J., Liu Q., Mendez J. A., Curiel T., Michalek J., Mahalingam D., Br. J. Cancer 2022, 127, 1153; - PMC - PubMed
- c) Boone B. A., Bahary N., Zureikat A. H., Moser A. J., Normolle D. P., Wu W.‐C., Singhi A. D., Bao P., Bartlett D. L., Liotta L. A., Espina V., Loughran P., Lotze M. T., H. J. Zeh 3rd, Ann Surg Oncol 2015, 22, 4402; - PMC - PubMed
- d) Brana I., Ocana A., Chen E. X., Razak A. R. A., Haines C., Lee C., Douglas S., Wang L., Siu L. L., Tannock I. F., Bedard P. L., Invest New Drugs 2014, 32, 1269; - PubMed
- e) Chi M.‐S., Lee C.‐Y., Huang S. u.‐C., Yang K.‐L., Ko H.‐L., Chen Y.‐K., Chung C.‐H., Liao K.‐W., Chi K.‐H., Oncotarget 2015, 6, 29808; - PMC - PubMed
- f) George M. A., Mayer T. M., Moore D., Chen C., White E., DiPaola R. S., Jeyamohan C., Stein M. N., J. Clin. Oncol. 2017, 35, 102;
- g) Haas N. B., Appleman L. J., Stein M., Redlinger M., Wilks M., Xu X., Onorati A., Kalavacharla A., Kim T., Zhen C. J., Kadri S., Segal J. P., Gimotty P. A., Davis L. E., Amaravadi R. K., Clin. Cancer Res. 2019, 25, 2080; - PMC - PubMed
- h) Hansen A. R., Tannock I. F., Templeton A., Chen E., Evans A., Knox J., Prawira A., Sridhar S. S., Tan S., Vera‐Badillo F., Wang L., Wouters B. G., Joshua A. M., Oncologist 2019, 24, 1188; - PMC - PubMed
- i) Karasic T. B., Brown T. J., Schneider C., Teitelbaum U. R., Reiss K. A., Mitchell T. C., Massa R. C., O'hara M. H., Dicicco L., Garcia‐Marcano L., Amaravadi R. K., O'dwyer P. J., Oncologist 2022, 27, 716; - PMC - PubMed
- j) Karasic T. B., O'hara M. H., Loaiza‐Bonilla A., Reiss K. A., Teitelbaum U. R., Borazanci E., De Jesus‐Acosta A., Redlinger C., Burrell J. A., Laheru D. A., Von Hoff D. D., Amaravadi R. K., Drebin J. A., O'dwyer P. J., JAMA Oncol 2019, 5, 993; - PMC - PubMed
- k) Mahalingam D., Mita M., Sarantopoulos J., Wood L., Amaravadi R. K., Davis L. E., Mita A. C., Curiel T. J., Espitia C. M., Nawrocki S. T., Giles F. J., Carew J. S., Autophagy 2014, 10, 1403; - PMC - PubMed
- l) Malhotra J., Jabbour S., Orlick M., Riedlinger G., Guo Y., White E., Aisner J., Cancer Treat Res Commun 2019, 21, 100158; - PubMed
- m) Rangwala R., Chang Y. C., Hu J., Algazy K. M., Evans T. L., Fecher L. A., Schuchter L. M., Torigian D. A., Panosian J. T., Troxel A. B., Tan K.‐S., Heitjan D. F., Demichele A. M., Vaughn D. J., Redlinger M., Alavi A., Kaiser J., Pontiggia L., Davis L. E., O'dwyer P. J., Amaravadi R. K., Autophagy 2014, 10, 1391; - PMC - PubMed
- n) Rosenfeld M. R., Ye X., Supko J. G., Desideri S., Grossman S. A., Brem S., Mikkelson T., Wang D., Chang Y. C., Hu J., Mcafee Q., Fisher J., Troxel A. B., Piao S., Heitjan D. F., Tan K.‐S., Pontiggia L., O'dwyer P. J., Davis L. E., Amaravadi R. K., Autophagy 2014, 10, 1359; - PMC - PubMed
- o) Wolpin B. M., Rubinson D. A., Wang X., Chan J. A., Cleary J. M., Enzinger P. C., Fuchs C. S., Mccleary N. J., Meyerhardt J. A., Ng K., Schrag D., Sikora A. L., Spicer B. A., Killion L., Mamon H., Kimmelman A. C., Oncologist 2014, 19, 637; - PMC - PubMed
- p) Zeh H. J., Bahary N., Boone B. A., Singhi A. D., Miller‐Ocuin J. L., Normolle D. P., Zureikat A. H., Hogg M. E., Bartlett D. L., Lee K. K., Tsung A., Marsh J. W., Murthy P., Tang D., Seiser N., Amaravadi R. K., Espina V., Liotta L., Lotze M. T., Clin. Cancer Res. 2020, 26, 3126. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
