Enzyme replacement therapy for late-onset Pompe disease
- PMID: 38084761
- PMCID: PMC10714667
- DOI: 10.1002/14651858.CD012993.pub2
Enzyme replacement therapy for late-onset Pompe disease
Abstract
Background: Pompe disease is caused by a deficiency of the enzyme acid alpha-glucosidase (GAA). People with infantile-onset disease have either a complete or a near-complete enzyme deficiency; people with late-onset Pompe disease (LOPD) retain some residual enzyme activity. GAA deficiency is treated with an intravenous infusion of recombinant human acid alglucosidase alfa, an enzyme replacement therapy (ERT). Alglucosidase alfa and avalglucosidase alfa are approved treatments, but cipaglucosidase alfa with miglustat is not yet approved.
Objectives: To assess the effects of enzyme replacement therapies in people with late-onset Pompe disease.
Search methods: We searched the Cochrane Inborn Errors of Metabolism Trials Register, compiled from electronic database searches and handsearching of journals and conference abstract books. We also searched MEDLINE OvidSP, clinical trial registries, and the reference lists of relevant articles and reviews. Date of last search: 21 April 2022.
Selection criteria: We included randomised controlled trials (RCTs) of ERT in people with LOPD of any age.
Data collection and analysis: Two review authors independently assessed trial eligibility, extracted data, assessed the risk of bias and the certainty of the evidence (using GRADE). We resolved disagreements through discussion and by consulting a third author.
Main results: We included six trials (358 randomised participants) lasting from 12 to 78 weeks. A single trial reported on each comparison listed below. None of the included trials assessed two of our secondary outcomes: need for respiratory support and use of a walking aid or wheelchair. Certainty of evidence was most commonly downgraded for selective reporting bias. Alglucosidase alfa versus placebo (90 participants) After 78 weeks, alglucosidase alfa probably improves the six-minute walk test (6MWT) distance compared to placebo (mean difference (MD) 30.95 metres, 95% confidence interval (CI) 7.98 to 53.92; moderate-certainty evidence) and probably improves respiratory function, measured as the change in per cent (%) predicted forced vital capacity (FVC) (MD 3.55, 95% CI 1.46 to 5.64; moderate-certainty evidence). There may be little or no difference between the groups in occurrence of infusion reactions (risk ratio (RR) 1.21, 95% CI 0.57 to 2.61; low-certainty evidence), quality of life physical component score (MD -1.36 points, 95% CI -5.59 to 2.87; low-certainty evidence), or adverse events (RR 0.94, 95% CI 0.64 to 1.39; low-certainty evidence). Alglucosidase alfa plus clenbuterol versus alglucosidase alfa plus placebo (13 participants) The evidence is very uncertain about the effect of alglucosidase alfa plus clenbuterol compared to alglucosidase alfa plus placebo on: change in 6MWT distance after 52 weeks (MD 34.55 metres, 95% CI-10.11 to 79.21; very low-certainty evidence) and change in % predicted FVC (MD -13.51%, 95% CI -32.44 to 5.41; very low-certainty evidence). This study did not measure infusion reactions, quality of life, and adverse events. Alglucosidase alfa plus albuterol versus alglucosidase alfa plus placebo (13 participants) The evidence is very uncertain about the effect of alglucosidase alfa plus albuterol compared to alglucosidase alfa plus placebo on: change in 6MWT distance after 52 weeks (MD 30.00 metres, 95% CI 0.55 to 59.45; very low-certainty evidence), change in % predicted FVC (MD -4.30%, 95% CI -14.87 to 6.27; very low-certainty evidence), and risk of adverse events (RR 0.67, 95% CI 0.38 to 1.18; very low-certainty evidence). This study did not measure infusion reactions and quality of life. VAL-1221 versus alglucosidase alfa (12 participants) Insufficient information was available about this trial to generate effect estimates measured at one year or later. Compared to alglucosidase alfa, VAL-1221 may increase or reduce infusion-associated reactions at three months, but the evidence is very uncertain (RR 2.80, 95% CI 0.18 to 42.80). This study did not measure quality of life and adverse events. Cipaglucosidase alfa plus miglustat versus alglucosidase alfa plus placebo (125 participants) Compared to alglucosidase alfa plus placebo, cipaglucosidase alfa plus miglustat may make little or no difference to: 6MWT distance at 52 weeks (MD 13.60 metres, 95% CI -2.26 to 29.46); infusion reactions (RR 0.94, 95% CI 0.49 to 1.80); quality of life scores for physical function (MD 1.70, 95% CI -2.13 to 5.53) and fatigue (MD -0.30, 95% CI -2.76 to 2.16); and adverse effects potentially related to treatment (RR 0.83, 95% CI 0.49 to 1.40) (all low-certainty evidence). Cipaglucosidase alfa plus miglustat probably improves % predicted FVC compared to alglucosidase alfa plus placebo (MD 3.10%, 95% CI 1.04 to 5.16; moderate-certainty evidence); however, it may make little or no change in % predicted sniff nasal inspiratory pressure (MD -0.06%, 95% CI -8.91 to 7.71; low-certainty evidence). Avalglucosidase alfa versus alglucosidase alfa (100 participants) After 49 weeks, avalglucosidase alfa probably improves 6MWT compared to alglucosidase alfa (MD 30.02 metres, 95% CI 1.84 to 58.20; moderate-certainty evidence). Avalglucosidase alfa probably makes little or no difference to % predicted FVC compared to alglucosidase alfa (MD 2.43%, 95% CI -0.08 to 4.94; moderate-certainty evidence). Avalglucosidase alfa may make little or no difference to infusion reactions (RR 0.78, 95% CI 0.42 to 1.45), quality of life (MD 0.77, 95% CI -2.09 to 3.63), or treatment-related adverse events (RR 0.92, 95% CI 0.61 to 1.40), all low-certainty evidence.
Authors' conclusions: One trial compared the effect of ERT to placebo in LOPD, showing that alglucosidase alfa probably improves 6MWT and respiratory function (both moderate-certainty evidence). Avalglucosidase alfa probably improves 6MWT compared with alglucosidase alfa (moderate-certainty evidence). Cipaglucosidase plus miglustat probably improves FVC compared to alglucosidase alfa plus placebo (moderate-certainty evidence). Other trials studied the adjunct effect of clenbuterol and albuterol along with alglucosidase alfa, with little to no evidence of benefit. No significant rise in adverse events was noted with all ERTs. The impact of ERT on some outcomes remains unclear, and longer RCTs are needed to generate relevant information due to the progressive nature of LOPD. Alternative resources, such as post-marketing registries, could capture some of this information.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Figures
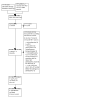




























Update of
References
References to studies included in this review
COMET 2021 {published data only}
-
- Berger K, DasMahapatra P, Baranowski E, Haack KA, Sparks S, Thibault N, et al. POMPE DISEASE: EP.200 patient relevant change in forced vital capacity % predicted in late onset Pompe disease (LOPD) in the COMET trial. Neuromuscular Disorders 2021;31 Suppl 1:S110.
-
- Diaz-Manera J, Kishnani PS, Kushlaf H, Ladha S, Mozaffar T, Straub V, et al. Safety and efficacy of avalglucosidase alfa versus alglucosidase alfa in patients with late-onset Pompe disease (COMET): a phase 3, randomised, multicentre trial. Lancet Neurology 2021;20(12):1012-26. - PubMed
-
- Kishnani P, Diaz-Manera J, Kushlaf H, Ladha S, Mozaffar T, Straub V, et al. The avalglucosidase alfa phase 3 COMET trial in late-onset Pompe disease patients: efficacy and safety results after 97 weeks. Molecular Genetics and Metabolism 2022;135(2):S66-7.
-
- Kushlaf H, Attarian S, Borges JL, Bouhour F, Chien Y-H, Choi Y-C, et al. Efficacy and safety results of the avalglucosidase alfa phase 3 COMET trial in late-onset pompe disease patients. Neurology 2021;96(15 Suppl 1):[no pagination].
-
- NCT02782741. Study to compare the efficacy and safety of enzyme replacement therapies avalglucosidase alfa and alglucosidase alfa administered every other week in patients with late-onset Pompe disease who have not been previously treated for pompe disease (COMET) [A phase 3 randomized, multicenter, multinational, double-blinded study comparing the efficacy and safety of repeated biweekly infusions of avalglucosidase alfa (neogaa, gz402666) and alglucosidase alfa in treatment naïve patients with late-onset Pompe disease]. clinicaltrials.gov/show/NCT02782741 (first posted 25 May 2016) (last accessed 26 July 2023).
Kishnani 2019 {published data only}
-
- EudraCT Number: 2016-004578-16. A three-month, open-label, randomized, dose-escalation study of the safety, tolerability, pharmacokinetics, pharmacodynamics and preliminary efficacy of VAL-1221 versus Myozyme®/Lumizyme® in patients with late-onset Pompe disease. clinicaltrialsregister.eu/ctr-search/search?query=2016-004578-16 (start date 19 June 2017) (last accessed 22 April 2022).
-
- Kishnani P, Lachmann R, Mozaffar T, Walters C, Case L, Appleby M, et al. Safety and efficacy of VAL-1221, a novel fusion protein targeting cytoplasmic glycogen, in patients with late-onset Pompe disease. Molecular Genetics and Metabolism 2019;126(2):S85-6. [DOI: ]
-
- NCT02898753. VAL-1221 delivered intravenously in ambulatory and ventilator-free participants with late-onset Pompe disease. clinicaltrials.gov/ct2/show/NCT02782741 (first posted 13 September 2016) (last accessed 22 April 2022).
Koeberl 2018 {published data only}
-
- Koeberl DD, Case LE, Smith EC, Walters C, Han S-O, Li Y, et al. Correction of biochemical abnormalities and gene expression associated with improved muscle function in a phase I/II clinical trial of clenbuterol in Pompe disease patients stably treated with ERT. Molecular Genetics and Metabolism 2018;123(2):S79-80.
-
- NCT01942590. Safety and efficacy of clenbuterol in individuals with late-onset Pompe disease and receiving enzyme replacement therapy [A clinical investigation of the safety and efficacy of clenbuterol on motor function in individuals with late-onset pompe disease and receiving enzyme replacement therapy]. clinicaltrials.gov/show/NCT01942590 (first posted 16 September 2013) (last accessed 22 April 2022).
Koeberl 2019 {published data only}
-
- Koeberl DD, Case LE, Desai A, Smith EC, Walters C, Han SO, et al. Improved muscle function in a phase I/II clinical trial of albuterol in Pompe disease. Molecular Genetics & Metabolism 2019;129(2):67-72. [PMID: ] - PubMed
-
- NCT01885936. Safety and efficacy of albuterol in individuals with late-onset Pompe disease [A phase 1/2 double-blind study of the safety and efficacy of albuterol on motor function in individuals with late-onset pompe disease receiving enzyme replacement therapy]. clinicaltrials.gov/show/NCT01885936 (first posted 25 June 2013) (last accessed 22 April 2022).
PROPEL 2021 {published data only}
-
- Byrne B, Bratkovic D, Diaz-Manera J, Laforet P, Mozaffar T, Ploeg A, et al. Cipaglucosidase alfa/miglustat versus alglucosidase alfa/placebo in late-onset Pompe disease (LOPD): PROPEL study subgroup analyses. Molecular Genetics and Metabolism 2022;135(2):S27-8.
-
- Chien Y-H, Benjamin E, Schoser B, Kishnani P, Mozaffar T, Diaz-Manera J, et al. Immunogenicity of cipaglucosidase alfa/miglustat versus alglucosidase alfa/placebo in late-onset Pompe disease (LOPD): a phase III, randomized study (PROPEL). Molecular Genetics and Metabolism 2022;135(2):S30.
-
- EudraCT Number: 2018-000755-40. A Phase 3 double-blind randomized study to assess the efficacy and safety of intravenous ATB200 co-administered with oral AT2221 in adult subjects with late onset Pompe disease compared with alglucosidase alfa/placebo. clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2018-00... (start date: 8 April 2019) (last accessed 26 July 2023).
-
- JapicCTI-194887. A phase 3 double-blind randomized study to assess the efficacy and safety of intravenous ATB200 co-administered with oral AT2221 in adult subjects with late- onset pompe disease compared with alglucosidase alfa/placebo. clinicaltrials.jp/user/showCteDetailE.jsp?japicId=JapicCTI-194887 (first posted 29 July 2019) (last accessed 26 July 2023).
-
- NCT03729362. PROPEL study - a study comparing ATB200/AT2221 with alglucosidase/placebo in adult subjects with LOPD [A phase 3 double-blind randomized study to assess the efficacy and safety of intravenous atb200 co-administered with oral at2221 in adult subjects with late onset Pompe disease compared with alglucosidase alfa/placebo]. clinicaltrials.gov/show/NCT03729362 (first posted 02 November 2018) (last accessed 26 July 2023).
Van der Ploeg 2010 {published data only}
-
- Ecker‐Schlipf B. Pompe disease: successful treatment with alglucosidase alfa in late onset of the disease [Morbus pompe: behandlungserfolge mit alglucosidase alfa bei der späten krankheitsform]. www.arzneimitteltherapie.de/heftarchiv/2010/10/behandlungserfolge-mit-al... 2010 (last accessed 26 July 2023);28(10):[no pagination].
-
- EudraCT Number: 2005-002759-42. A randomized, double-blind, multicenter, multinational, placebo-controlled study of the safety, efficacy, and pharmacokinetics of myozyme, recombinant human acid alpha-glucosidase (rhGAA), treatment in patients with late-onset Pompe disease. www.clinicaltrialsregister.eu/ctr-search/trial/2005-002759-42/DE (first registered 24 July 2009) (last accessed 22 April 2022).
-
- Laforet P, Clemens PR, Corzo D, Escolar D, Florence J, Van der Ploeg A, et al. Safety and efficacy results from a randomized, double-blind, placebo-controlled study of alglucosidase alfa for the treatment of pompe disease in juveniles and adults. Neuromuscular Disorders : NMD 2008;18(9-10):832-3.
-
- NCT00158600. A placebo-controlled study of safety and effectiveness of myozyme (alglucosidase alfa) in patients with late-onset Pompe disease [Randomized, double-blind, placebo-controlled study of the safety, efficacy and pharmacokinetics of myozyme in patients with late-onset Pompe disease]. clinicaltrials.gov/show/NCT00158600 (first posted 12 September 2005) (last accessed 26 July 2023).
References to studies excluded from this review
Case 2015 {published data only}
-
- NCT00483379. High dose or high dose frequency study of alglucosidase alfa [An exploratory, open-label study of the safety and efficacy of high dose or high dosing frequency alglucosidase alfa treatment in patients with Pompe disease who do not have an optimal response to the standard dose regimen]. clinicaltrials.gov/show/NCT00483379 (first posted 07 June 2007) (last accessed 22 April 2022).
Harlaar 2019 {published data only}
INSPIRE 2015 {published data only}
-
- Geberhiwot T, Byrne B, Eyskens F, Hughes D, Kissel J, Mengel E, et al. An international, phase 3, switchover study of reveglucosidase alfa (BMN 701) in subjects with late-onset pompe disease (INSPIRE study). Journal of Inherited Metabolic Disease 2015;38(1 Suppl):[no pagination]. [ABSTRACT NO.: O-055]
Kishnani 2009 {published data only}
Kronn 2022 {published data only}
-
- Kronn D, Davison J, Brassier A, Broomfield A, Hahn SH, Kumada S, et al. Mini-COMET study: safety, biomarker, and efficacy data after avalglucosidase alfa dosing for ≥ 97 weeks in participants with infantile-onset pompe disease (IOPD) previously treated with alglucosidase alfa who had demonstrated clinical decline. Molecular Genetics and Metabolism 2022;135(2):S68.
NCT04094948 {published data only}
-
- NCT04094948. Phase II clinical trial of clenbuterol in adult patients with Pompe disease. clinicaltrials.gov/ct2/show/NCT04094948 (first posted 2019) (last accessed 22 April 2022).
Additional references
Amato 2011
-
- Amato AA, Leep Hunderfund AN, Selcen D, Keegan BM. A 49-year-old woman with progressive shortness of breath. Neurology 2011;76(9):830-6. - PubMed
Ausems 1999
-
- Ausems MG, Verbiest J, Hermans MP, Kroos MA, Beemer FA, Wokke JH, et al. Frequency of glycogen storage disease type II in the Netherlands: implications for diagnosis and genetic counselling. European Journal of Human Genetics 1999;7(6):713-6. - PubMed
Beltran Papsdorf 2014
Berli 2021
-
- Berli S, Brandi G, Keller E, Najia N, Vitale J, Pagnamenta A. Clinical efficacy of the enzyme replacement therapy in patients with late-onset Pompe disease: a systematic review and a meta-analysis. Journal of Neurology 2021 Apr 13 [Epub ahead of print] (last accessed 22 April 2022). [DOI: 10.1007/s00415-021-10526-5] - DOI - PMC - PubMed
Chaimani 2022
-
- Chaimani A, Caldwell DM, Li T, Higgins JPT, Salanti G. Chapter 11: Undertaking network meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook (last accessed 22 April 2022).
Cohen 1960
-
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement 1960;20(1):37-46.
Deeks 2022
-
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook (accessed 22 November 2022).
EMA 2011
-
- EMA 2011. Annex 1. Summary of product characteristics. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information... (accessed 13 July 2017).
EMA 2022
-
- EMA 2022. Annex 1. Summary of product characteristics. www.ema.europa.eu/en/documents/product-information/nexviadyme-epar-produ... (accessed 12 September 2022).
Hagemans 2005
-
- Hagemans ML, Winkel LP, Van Doorn PA, Hop WJ, Loonen MC, Reuser AJ, et al. Clinical manifestation and natural course of late-onset Pompe's disease in 54 Dutch patients. Brain 2005;128(Pt 3):671-7. - PubMed
Higgins 2003
Higgins 2008
Higgins 2011
-
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/ (last accessed 26 July 2023).
Higgins 2023
-
- Higgins JPT, Li T, Deeks JJ (editors). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023.
Hirschorn 2001
-
- Hirschorn R, Reuser AJ. Glycogen storage disease type II: acid alpha-glucosidase (acid maltase) deficiency. In: Scriver CR, Valle D, editors(s). The Metabolic and Molecular Bases of Inherited Disease. 8th edition. New York (NY): McGraw-Hill, 2001:3389-420.
Johnson 2016
-
- Johnson EM, Roberts M, Mozaffar T, Young P, Quartel A, Berger KI. Pulmonary function tests (maximum inspiratory pressure, maximum expiratory pressure, vital capacity, forced vital capacity) predict ventilator use in late-onset Pompe disease. Neuromuscular Disorders 2016;26(2):136-45. - PubMed
Kishnani 2006
-
- Kishnani PS, Hwu WL, Mandel H, Nicolino M, Yong F, Corzo D, et al. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. Journal of Pediatrics 2006;148(5):671-6. - PubMed
Kishnani 2013
-
- Kishnani PS, Amartino HM, Lindberg C, Miller TM, Wilson A, Keutzer J, et al. Timing of diagnosis of patients with Pompe disease: data from the Pompe registry. American Journal of Medical Genetics 2013;161A(10):2431-43. - PubMed
Li 2023
-
- Li T, Higgins JPT, Deeks JJ (editors). Chapter 5: Collecting data. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023.
Mah 2010
Mechtler 2012
-
- Mechtler TP, Stary S, Metz TF, De Jesús VR, Greber-Platzer S, Pollak A, et al. Neonatal screening for lysosomal storage disorders: feasibility and incidence from a nationwide study in Austria. Lancet 2012;379(9813):335-41. - PubMed
Milverton 2019
-
- Milverton J, Newton S, Merlin T. The effectiveness of enzyme replacement therapy for juvenile-onset Pompe disease: a systematic review. Journal of Inherited Metabolic Disease 2019;42(1):57-65. - PubMed
Page 2022
-
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook (last accessed 22 April 2022).
Pellegrini 2005
-
- Pellegrini N, Laforet P, Orlikowski D, Pellegrini M, Caillaud C, Eymard B, et al. Respiratory insufficiency and limb muscle weakness in adults with Pompe's disease. European Respiratory Journal 2005;26(6):1024-31. - PubMed
Piraud 2020
-
- Piraud M, Pettazzoni M, Antonio M, Vianey-Saban C, Froissart R, Chabrol B, et al. Urine glucose tetrasaccharide: A good biomarker for glycogenoses type II and III? A study of the French cohort. Molecular Genetics and Metabolism Reports 2020;23:100583. [DOI: 10.1016/j.ymgmr.2020.100583] - DOI - PMC - PubMed
RevMan Web 2020 [Computer program]
-
- Review Manager Web (RevMan Web). Version 1.22.0. The Cochrane Collaboration, 2020. Available at: revman.cochrane.org.
Saville 2021
Schünemann 2022a
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook (last accessed 26 July 2023).
Schünemann 2022b
-
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook (last accessed 22 April 2022).
Stepien 2016
Tein 1996
-
- Tein I. Metabolic myopathies. Seminars in Pediatric Hematology 1996;3(2):59-98. - PubMed
Toscano 2013
-
- Toscano A, Schoser B. Enzyme replacement therapy in late-onset Pompe disease: a systematic literature review. Journal of Neurology 2013;260(4):951-9. - PubMed
Van den Hout 2003
-
- Van den Hout HM, Hop W, Van Diggelen OP, Smeitink JA, Smit GP, Poll-The BT, et al. The natural course of infantile Pompe's disease: 20 original cases compared with 133 cases from the literature. Pediatrics 2003;112(2):332-40. - PubMed
Van der Ploeg 2008
-
- Van der Ploeg AT, Reuser AJ. Pompe's disease. Lancet 2008;372(9646):1342-53. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

