HIV-1 capsid and viral DNA integration
- PMID: 38085100
- PMCID: PMC10790781
- DOI: 10.1128/mbio.00212-22
HIV-1 capsid and viral DNA integration
Abstract
HIV-1 capsid protein (CA)-independently or by recruiting host factors-mediates several key steps of virus replication in the cytoplasm and nucleus of the target cell. Research in the recent years have established that CA is multifunctional and genetically fragile of all the HIV-1 proteins. Accordingly, CA has emerged as a validated and high priority therapeutic target, and the first CA-targeting antiviral drug was recently approved for treating multi-drug resistant HIV-1 infection. However, development of next generation CA inhibitors depends on a better understanding of CA's known roles, as well as probing of CA's novel roles, in HIV-1 replication. In this timely review, we present an updated overview of the current state of our understanding of CA's multifunctional role in HIV-1 replication-with a special emphasis on CA's newfound post-nuclear roles, highlight the pressing knowledge gaps, and discuss directions for future research.
Keywords: HIV; capsid; integration; nuclear entry; reverse transcription.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
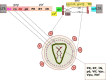
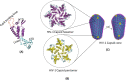
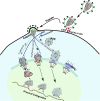
References
-
- UNAIDS . 2023. FACT sheet 2023 global HIV statistics
-
- Ostrowski MA, Chun TW, Justement SJ, Motola I, Spinelli MA, Adelsberger J, Ehler LA, Mizell SB, Hallahan CW, Fauci AS. 1999. Both memory and CD45RA+/CD62L+ naive CD4(+) T cells are infected in human immunodeficiency virus type 1-infected individuals. J Virol 73:6430–6435. doi:10.1128/JVI.73.8.6430-6435.1999 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
