Iopamidol Abatement from Waters: A Rigorous Approach to Determine Physicochemical Parameters Needed to Scale Up from Batch to Continuous Operation
- PMID: 38085695
- PMCID: PMC10753885
- DOI: 10.1021/acs.langmuir.3c02992
Iopamidol Abatement from Waters: A Rigorous Approach to Determine Physicochemical Parameters Needed to Scale Up from Batch to Continuous Operation
Abstract
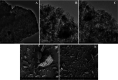
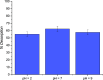
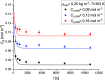
The abatement of iopamidol (IPM), an X-ray iodinated contrast agent, in aqueous solution using powdered activated carbon (PAC) as a sorbent was investigated in the present work. The material was characterized by various analytical techniques such as thermogravimetric analysis, scanning electron microscopy, transmission electron microscopy, Brunauer-Emmett-Teller analysis, dynamic light scattering, and zeta potential measurements. Both thermodynamic and kinetic experiments were conducted in a batch apparatus, and the effects of the initial concentration of IPM, the temperature, and the adsorbent bulk density on the adsorption kinetics were investigated. The adsorption isotherms were interpreted well using the Langmuir model. Moreover, it was demonstrated that IPM adsorption on PAC is spontaneous and exothermic (ΔH0 = -27 kJ mol-1). The adsorption kinetic data were described using a dynamic intraparticle model for fluid-solid adsorption kinetics (ADIM) allowing determination of a surface activation energy Es = 6 ± 1 kJ mol-1. Comparing the experimental results and the model predictions, a good model fit was obtained.
Conflict of interest statement
The authors declare no competing financial interest.
Figures











References
-
- Al-Baldawi I. A.; Mohammed A. A.; Mutar Z. H.; Abdullah S. R. S.; Jasim S. S.; Almansoory A. F.; Ismail N. I. Application of phytotechnology in alleviating pharmaceuticals and personal care products (PPCPs) in wastewater: Source, impacts, treatment, mechanisms, fate, and SWOT analysis. J. Cleaner Prod. 2021, 319, 128584.10.1016/j.jclepro.2021.128584. - DOI
-
- Hou M.; Li X.; Fu Y.; Wang L.; Lin D.; Wang Z. Degradation of Iodinated X-Ray Contrast Media by Advanced Oxidation Processes: A Literature Review with a Focus on Degradation Pathways. Chin. Chem. Lett. 2023, 34, 107723.10.1016/j.cclet.2022.08.003. - DOI
-
- Arslan-Alaton I.; Olmez-Hanci T.; Korkmaz G.; Sahin C. Removal of Iopamidol, an Iodinated X-Ray Contrast Medium, by Zero-Valent Aluminum-Activated H2O2 and S2O82-. Chem. Eng. J. 2017, 318, 64–75. 10.1016/j.cej.2016.05.021. - DOI
LinkOut - more resources
Full Text Sources

