Advances and applications of microcrystal electron diffraction (MicroED)
- PMID: 38086321
- PMCID: PMC10882645
- DOI: 10.1016/j.sbi.2023.102741
Advances and applications of microcrystal electron diffraction (MicroED)
Abstract
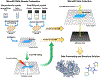
Microcrystal electron diffraction, commonly referred to as MicroED, has become a powerful tool for high-resolution structure determination. The method makes use of cryogenic transmission electron microscopes to collect electron diffraction data from crystals that are several orders of magnitude smaller than those used by other conventional diffraction techniques. MicroED has been used on a variety of samples including soluble proteins, membrane proteins, small organic molecules, and materials. Here we will review the MicroED method and highlight recent advancements to the methodology, as well as describe applications of MicroED within the fields of structural biology and chemical crystallography.
Keywords: Cryo-EM; Cryo-electron microscopy; Crystallography; MicoED; Microcrystal electron diffraction; Protein crystallography; Small molecule crystallography.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
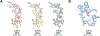
References
-
- Henderson R: The potential and limitations of neutrons, electrons and X-rays for atomic resolution microscopy of unstained biological molecules. Q Rev Biophys 1995, 28:171–193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

