Targeted design of synthetic enhancers for selected tissues in the Drosophila embryo
- PMID: 38086418
- PMCID: PMC10830412
- DOI: 10.1038/s41586-023-06905-9
Targeted design of synthetic enhancers for selected tissues in the Drosophila embryo
Abstract
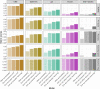
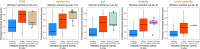
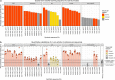
Enhancers control gene expression and have crucial roles in development and homeostasis1-3. However, the targeted de novo design of enhancers with tissue-specific activities has remained challenging. Here we combine deep learning and transfer learning to design tissue-specific enhancers for five tissues in the Drosophila melanogaster embryo: the central nervous system, epidermis, gut, muscle and brain. We first train convolutional neural networks using genome-wide single-cell assay for transposase-accessible chromatin with sequencing (ATAC-seq) datasets and then fine-tune the convolutional neural networks with smaller-scale data from in vivo enhancer activity assays, yielding models with 13% to 76% positive predictive value according to cross-validation. We designed and experimentally assessed 40 synthetic enhancers (8 per tissue) in vivo, of which 31 (78%) were active and 27 (68%) functioned in the target tissue (100% for central nervous system and muscle). The strategy of combining genome-wide and small-scale functional datasets by transfer learning is generally applicable and should enable the design of tissue-, cell type- and cell state-specific enhancers in any system.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures









References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

