A molecular switch for neuroprotective astrocyte reactivity
- PMID: 38086421
- PMCID: PMC11384621
- DOI: 10.1038/s41586-023-06935-3
A molecular switch for neuroprotective astrocyte reactivity
Abstract
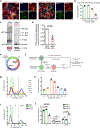
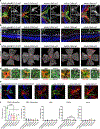
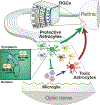
The intrinsic mechanisms that regulate neurotoxic versus neuroprotective astrocyte phenotypes and their effects on central nervous system degeneration and repair remain poorly understood. Here we show that injured white matter astrocytes differentiate into two distinct C3-positive and C3-negative reactive populations, previously simplified as neurotoxic (A1) and neuroprotective (A2)1,2, which can be further subdivided into unique subpopulations defined by proliferation and differential gene expression signatures. We find the balance of neurotoxic versus neuroprotective astrocytes is regulated by discrete pools of compartmented cyclic adenosine monophosphate derived from soluble adenylyl cyclase and show that proliferating neuroprotective astrocytes inhibit microglial activation and downstream neurotoxic astrocyte differentiation to promote retinal ganglion cell survival. Finally, we report a new, therapeutically tractable viral vector to specifically target optic nerve head astrocytes and show that raising nuclear or depleting cytoplasmic cyclic AMP in reactive astrocytes inhibits deleterious microglial or macrophage cell activation and promotes retinal ganglion cell survival after optic nerve injury. Thus, soluble adenylyl cyclase and compartmented, nuclear- and cytoplasmic-localized cyclic adenosine monophosphate in reactive astrocytes act as a molecular switch for neuroprotective astrocyte reactivity that can be targeted to inhibit microglial activation and neurotoxic astrocyte differentiation to therapeutic effect. These data expand on and define new reactive astrocyte subtypes and represent a step towards the development of gliotherapeutics for the treatment of glaucoma and other optic neuropathies.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures


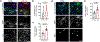





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

