Tolerability Outcomes of American Thoracic Society/Infectious Diseases Society of America Guideline-Recommended Multidrug Antibiotic Treatment for Mycobacterium avium Complex Pulmonary Disease in US Medicare Beneficiaries With Bronchiectasis
- PMID: 38086472
- PMCID: PMC11214905
- DOI: 10.1016/j.chest.2023.12.006
Tolerability Outcomes of American Thoracic Society/Infectious Diseases Society of America Guideline-Recommended Multidrug Antibiotic Treatment for Mycobacterium avium Complex Pulmonary Disease in US Medicare Beneficiaries With Bronchiectasis
Abstract
Background: Nontuberculous mycobacteria are environmental organisms that are increasingly causing chronic and debilitating pulmonary infections, of which Mycobacterium avium complex (MAC) is the most common pathogen. MAC pulmonary disease (MAC-PD) is often difficult to treat, often requiring long-term multidrug antibiotic therapy.
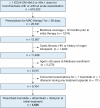
Research question: Is there an association between various guideline-based three-drug therapy (GBT) regimens and (1) therapy-associated adverse events or (2) regimen change/discontinuation, within 12 months of therapy initiation?
Study design and methods: In a retrospective cohort study, we examined tolerability outcomes of GBT regimens for MAC-PD in 4,626 US Medicare beneficiaries with bronchiectasis, who were prescribed a GBT as initial antibiotic treatment for presumed MAC-PD during 2006 to 2014. Using multivariable Cox proportional hazard regression, we estimated adjusted hazard ratios (aHRs) to compare the risk of adverse events and regimen change/discontinuations within 12 months of therapy initiation in various GBT regimens.
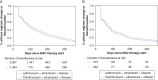
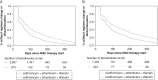
Results: The cohort had a mean age ± SD of 77.9 ± 6.1 years at treatment start, were mostly female (77.7%), and were mostly non-Hispanic White (87.2%). The risk of regimen change/discontinuation within 12 months of therapy was higher for clarithromycin-based regimens than azithromycin-based regimens (aHR, 1.12; 95% CI, 1.04-1.20 with rifampin; aHR, 1.11; 95% CI, 0.93-1.32 with rifabutin as the companion rifamycin), and for rifabutin-containing regimens than rifampin-containing regimens (aHR, 1.49; 95% CI, 1.33-1.68 with azithromycin; aHR, 1.47; 95% CI, 1.27-1.70 with clarithromycin as the companion macrolide). The aHR comparing regimen change/discontinuation with clarithromycin-ethambutol-rifabutin and azithromycin-ethambutol-rifampin was 1.64 (95% CI, 1.43-1.64).
Interpretation: Overall, an azithromycin-based regimen was less likely to be changed or discontinued than a clarithromycin-based regimen, and a rifampin-containing regimen was less likely to be changed or discontinued than a rifabutin-containing regimen within 12 months of therapy start. Our work provides a population-based assessment on the tolerability of multidrug antibiotic regimens used for the treatment of MAC-PD.
Keywords: Medicare; Medicare claims; Mycobacterium avium complex; antibiotic therapy; nontuberculous mycobacterial infection.
Copyright © 2023 American College of Chest Physicians. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Financial/Nonfinancial Disclosures The authors have reported to CHEST the following: E. H. has served on an advisory board for AN2 pharmaceuticals and received consult fees. K. L. W. has grants or contracts from Insmed; and has received consulting fees from Insmed, Paratek, RedHill Biopharma, Spero Therapeutics, and AN2 pharmaceuticals. T. K. M. has grants or contracts from Insmed, the Centered Outcomes Research Institute, and the Lung Health Foundation; has received consulting fees from Insmed, RedHill Biopharma, and Spero Therapeutics; has received payment or honoraria from France Foundation, Astra Zeneca, Novartis, and Insmed; and has a leadership or fiduciary role in the Toronto NTM patient group. None declared (J. H. K., K. F. C., M. M., S. K. B.).
Figures


References
-
- Falkinham J.O., III Surrounded by mycobacteria: nontuberculous mycobacteria in the human environment. J Appl Microbiol. 2009;107(2):356–367. - PubMed
-
- Griffith D.E., Aksamit T., Brown-Elliott B.A., et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. - PubMed
-
- Egelund E.F., Fennelly K.P., Peloquin C.A. Medications and monitoring in nontuberculous mycobacteria infections. Clin Chest Med. 2015;36(1):55–66. - PubMed
-
- O'Brien R.J., Geiter L.J., Snider D.E., Jr. The epidemiology of nontuberculous mycobacterial diseases in the United States. Results from a national survey. Am Rev Respir Dis. 1987;135(5):1007–1014. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

