Programmed cell death in hepatic fibrosis: current and perspectives
- PMID: 38086792
- PMCID: PMC10716404
- DOI: 10.1038/s41420-023-01749-8
Programmed cell death in hepatic fibrosis: current and perspectives
Abstract
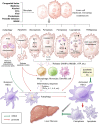
The initiation, development and resolution of hepatic fibrosis are influenced by various cytokines, chemokines, damage-associated molecular patterns (DAMPs) and signaling pathways. A significant number of studies in recent years have indicated that the progression of hepatic fibrosis is closely linked to programmed cell death processes such as apoptosis, autophagy, pyroptosis, necroptosis, ferroptosis, cuproptosis, and PANoptosis. Inducement of hepatic stellate cells (HSCs) death or preventing death in other liver cells can delay or even reverse hepatic fibrosis. Nevertheless, the roles of programmed cell death in hepatic fibrosis have not been reviewed. Therefore, this review summarizes the characteristics of various of hepatic fibrosis and programmed cell death, focuses on the latest progress of programmed cell death in the promotion and regression of hepatic fibrosis, and highlights the different roles of the programmed cell death of HSCs and other liver cells in hepatic fibrosis. In the end, the possible therapeutic approaches targeting programmed cell death for treating hepatic fibrosis are discussed and prospected.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

