The development of a hiPSC-based platform to identify tissue-dependencies of IDH1 R132H
- PMID: 38086797
- PMCID: PMC10716401
- DOI: 10.1038/s41420-023-01747-w
The development of a hiPSC-based platform to identify tissue-dependencies of IDH1 R132H
Abstract
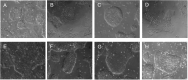
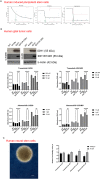
The application of patient-derived (PD) in vitro tumor models represents the classical strategy for clinical translational oncology research. Using these cellular heterogeneous cultures for the isolation of cancer stem cells (CSCs), suggested to be the main driver for disease malignancy, relies on the use of surrogate biomarkers or is based on CSC-enriching culture conditions. However, the ability of those strategies to exclusively and efficiently enrich for CSC pool has been questioned. Here we present an alternative in vitro CSC model based on the oncogenic transformation of single clone-derived human induced pluripotent stem cells (hiPSC). Hotspot mutations in the DNA encoding for the R132 codon of the enzyme isocitrate dehydrogenase 1 (IDH1) and codon R175 of p53 are commonly occurring molecular features of different tumors and were selected for our transformation strategy. By choosing p53 mutant glial tumors as our model disease, we show that in vitro therapy discovery tests on IDH1-engineered synthetic CSCs (sCSCs) can identify kinases-targeting chemotherapeutics that preferentially target tumor cells expressing corresponding genetic alteration. In contrast, neural stem cells (NSCs) derived from the IDH1R132H overexpressing hiPSCs increase their resistance to the tested interventions indicating glial-to-neural tissue-dependent differences of IDH1R132H. Taken together, we provide proof for the potential of our sCSC technology as a potent addition to biomarker-driven drug development projects or studies on tumor therapy resistance. Moreover, follow-up projects such as comparing in vitro drug sensitivity profiles of hiPSC-derived tissue progenitors of different lineages, might help to understand a variety of tissue-related functions of IDH1 mutations.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

