Chemokine CCL2 promotes cardiac regeneration and repair in myocardial infarction mice via activation of the JNK/STAT3 axis
- PMID: 38086898
- PMCID: PMC10943228
- DOI: 10.1038/s41401-023-01198-0
Chemokine CCL2 promotes cardiac regeneration and repair in myocardial infarction mice via activation of the JNK/STAT3 axis
Erratum in
-
Correction: Chemokine CCL2 promotes cardiac regeneration and repair in myocardial infarction mice via activation of the JNK/STAT3 axis.Acta Pharmacol Sin. 2025 May;46(5):1494. doi: 10.1038/s41401-024-01447-w. Acta Pharmacol Sin. 2025. PMID: 39643642 Free PMC article. No abstract available.
Abstract
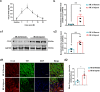
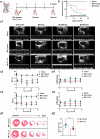
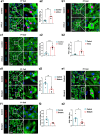
Stimulation of adult cardiomyocyte proliferation is a promising strategy for treating myocardial infarction (MI). Earlier studies have shown increased CCL2 levels in plasma and cardiac tissue both in MI patients and mouse models. In present study we investigated the role of CCL2 in cardiac regeneration and the underlying mechanisms. MI was induced in adult mice by permanent ligation of the left anterior descending artery, we showed that the serum and cardiac CCL2 levels were significantly increased in MI mice. Intramyocardial injection of recombinant CCL2 (rCCL2, 1 μg) immediately after the surgery significantly promoted cardiomyocyte proliferation, improved survival rate and cardiac function, and diminished scar sizes in post-MI mice. Alongside these beneficial effects, we observed an increased angiogenesis and decreased cardiomyocyte apoptosis in post-MI mice. Conversely, treatment with a selective CCL2 synthesis inhibitor Bindarit (30 μM) suppressed both CCL2 expression and cardiomyocyte proliferation in P1 neonatal rat ventricle myocytes (NRVMs). We demonstrated in NRVMs that the CCL2 stimulated cardiomyocyte proliferation through STAT3 signaling: treatment with rCCL2 (100 ng/mL) significantly increased the phosphorylation levels of STAT3, whereas a STAT3 phosphorylation inhibitor Stattic (30 μM) suppressed rCCL2-induced cardiomyocyte proliferation. In conclusion, this study suggests that CCL2 promotes cardiac regeneration via activation of STAT3 signaling, underscoring its potential as a therapeutic agent for managing MI and associated heart failure.
Keywords: Bindarit; CCL2; PF-4136309; STAT3; cardiomyocyte proliferation; myocardial infarction; neonatal rat ventricle myocytes; stattic.
© 2023. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Reed GW, Rossi JE, Cannon CP. Acute myocardial infarction. Lancet. 2017;389:197–210. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

