Acute irradiation induces a senescence-like chromatin structure in mammalian oocytes
- PMID: 38086992
- PMCID: PMC10716162
- DOI: 10.1038/s42003-023-05641-0
Acute irradiation induces a senescence-like chromatin structure in mammalian oocytes
Abstract
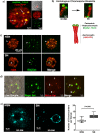
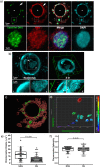
The mechanisms leading to changes in mesoscale chromatin organization during cellular aging are unknown. Here, we used transcriptional activator-like effectors, RNA-seq and superresolution analysis to determine the effects of genotoxic stress on oocyte chromatin structure. Major satellites are organized into tightly packed globular structures that coalesce into chromocenters and dynamically associate with the nucleolus. Acute irradiation significantly enhanced chromocenter mobility in transcriptionally inactive oocytes. In transcriptionally active oocytes, irradiation induced a striking unfolding of satellite chromatin fibers and enhanced the expression of transcripts required for protection from oxidative stress (Fermt1, Smg1), recovery from DNA damage (Tlk2, Rad54l) and regulation of heterochromatin assembly (Zfp296, Ski-oncogene). Non-irradiated, senescent oocytes exhibit not only high chromocenter mobility and satellite distension but also a high frequency of extra chromosomal satellite DNA. Notably, analysis of biological aging using an oocyte-specific RNA clock revealed cellular communication, posttranslational protein modifications, chromatin and histone dynamics as the top cellular processes that are dysregulated in both senescent and irradiated oocytes. Our results indicate that unfolding of heterochromatin fibers following acute genotoxic stress or cellular aging induced the formation of distended satellites and that abnormal chromatin structure together with increased chromocenter mobility leads to chromosome instability in senescent oocytes.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

