Benefits, harms and cost-effectiveness of cervical screening, triage and treatment strategies for women in the general population
- PMID: 38087115
- PMCID: PMC10719104
- DOI: 10.1038/s41591-023-02600-4
Benefits, harms and cost-effectiveness of cervical screening, triage and treatment strategies for women in the general population
Abstract
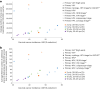
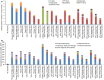
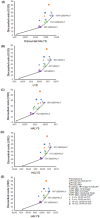
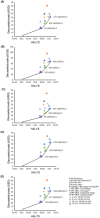
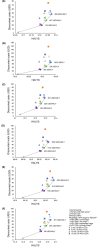
In 2020, the World Health Organization (WHO) launched a strategy to eliminate cervical cancer as a public health problem. To support the strategy, the WHO published updated cervical screening guidelines in 2021. To inform this update, we used an established modeling platform, Policy1-Cervix, to evaluate the impact of seven primary screening scenarios across 78 low- and lower-middle-income countries (LMICs) for the general population of women. Assuming 70% coverage, we found that primary human papillomavirus (HPV) screening approaches were the most effective and cost-effective, reducing cervical cancer age-standardized mortality rates by 63-67% when offered every 5 years. Strategies involving triaging women before treatment (with 16/18 genotyping, cytology, visual inspection with acetic acid (VIA) or colposcopy) had close-to-similar effectiveness to HPV screening without triage and fewer pre-cancer treatments. Screening with VIA or cytology every 3 years was less effective and less cost-effective than HPV screening every 5 years. Furthermore, VIA generated more than double the number of pre-cancer treatments compared to HPV. In conclusion, primary HPV screening is the most effective, cost-effective and efficient cervical screening option in LMICs. These findings have directly informed WHO's updated cervical screening guidelines for the general population of women, which recommend primary HPV screening in a screen-and-treat or screen-triage-and-treat approach, starting from age 30 years with screening every 5 years or 10 years.
© 2023. The Author(s).
Conflict of interest statement
K.S. received salary support from the Cancer Institute NSW (Australia, grant no. CDF1004). M.A. was supported by the Horizon 2020 Framework Programme for Research and Innovation of the European Commission, through the RISCC Network (grant no. 847845). K.C. receives salary support from the National Health and Medical Research Council (Australia, grant no. APP1135172). K.C. is co-principal investigator and M.C. is an investigator on an investigator-initiated trial of cytology and primary HPV screening in Australia (‘COMPASS’) (
Figures








References
-
- Ferlay, J et al. Global Cancer Observatory: Cancer Today. International Agency for Research on Cancer (Lyon, France). https://gco.iarc.fr/today (2020).
-
- World Health Organization. WHO Director-General calls for all countries to take action to help end the suffering caused by cervical cancer. https://www.who.int/reproductivehealth/call-to-action-elimination-cervic... (2019).
-
- World Health Organization. Global strategy to accelerate the elimination of cervical cancer as a public health problem. https://www.who.int/publications/i/item/9789240014107 (2020).

