Evidence of brain target engagement in Parkinson's disease and multiple sclerosis by the investigational nanomedicine, CNM-Au8, in the REPAIR phase 2 clinical trials
- PMID: 38087362
- PMCID: PMC10717868
- DOI: 10.1186/s12951-023-02236-z
Evidence of brain target engagement in Parkinson's disease and multiple sclerosis by the investigational nanomedicine, CNM-Au8, in the REPAIR phase 2 clinical trials
Erratum in
-
Correction: Evidence of brain target engagement in Parkinson's disease and multiple sclerosis by the investigational nanomedicine, CNM- Au8, in the REPAIR phase 2 clinical trials.J Nanobiotechnology. 2024 Jan 3;22(1):16. doi: 10.1186/s12951-023-02269-4. J Nanobiotechnology. 2024. PMID: 38167088 Free PMC article. No abstract available.
Abstract
Background: Impaired brain energy metabolism has been observed in many neurodegenerative diseases, including Parkinson's disease (PD) and multiple sclerosis (MS). In both diseases, mitochondrial dysfunction and energetic impairment can lead to neuronal dysfunction and death. CNM-Au8® is a suspension of faceted, clean-surfaced gold nanocrystals that catalytically improves energetic metabolism in CNS cells, supporting neuroprotection and remyelination as demonstrated in multiple independent preclinical models. The objective of the Phase 2 REPAIR-MS and REPAIR-PD clinical trials was to investigate the effects of CNM-Au8, administered orally once daily for twelve or more weeks, on brain phosphorous-containing energy metabolite levels in participants with diagnoses of relapsing MS or idiopathic PD, respectively.
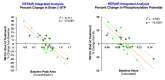
Results: Brain metabolites were measured using 7-Tesla 31P-MRS in two disease cohorts, 11 participants with stable relapsing MS and 13 participants with PD (n = 24 evaluable post-baseline scans). Compared to pre-treatment baseline, the mean NAD+/NADH ratio in the brain, a measure of energetic capacity, was significantly increased by 10.4% after 12 + weeks of treatment with CNM-Au8 (0.584 units, SD: 1.3; p = 0.037, paired t-test) in prespecified analyses of the combined treatment cohorts. Each disease cohort concordantly demonstrated increases in the NAD+/NADH ratio but did not reach significance individually (p = 0.11 and p = 0.14, PD and MS cohorts, respectively). Significant treatment effects were also observed for secondary and exploratory imaging outcomes, including β-ATP and phosphorylation potential across both cohorts.
Conclusions: Our results demonstrate brain target engagement of CNM-Au8 as a direct modulator of brain energy metabolism, and support the further investigation of CNM-Au8 as a potential disease modifying drug for PD and MS.
Keywords: Brain energy metabolites; Catalytic gold nanotherapeutic; Drug development; Magnetic resonance spectroscopy; Multiple sclerosis; Neuroimaging; Parkinson’s Disease; Target engagement.
© 2023. The Author(s).
Conflict of interest statement
JR, RBDIII, and RBD Jr have no competing interests to declare. AR, JaE, JeE, KSH, RG, MTH and BMG are employees of Clene and own stock in the company. At the time of study conductance, BMG was solely affiliated with UTSW. He was employed by Clene as a consultant after the conclusion of the described studies. SL is an employee of the contract research organization, Instat. PS has no disclosures related to this publication. He has received consulting fees from Medical Logix LLC, Genentech, and Bristol Myers Squibb.
Figures

References
-
- Mattson MP, Pedersen WA, Duan W, Culmsee C, Camandola S. Cellular and molecular mechanisms underlying perturbed energy metabolism and neuronal degeneration in Alzheimer’s and Parkinson’s Diseases. Ann N Y Acad Sci. 1999;893:154–75. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

