Transgelin Promotes Glioblastoma Stem Cell Hypoxic Responses and Maintenance Through p53 Acetylation
- PMID: 38087889
- PMCID: PMC10870072
- DOI: 10.1002/advs.202305620
Transgelin Promotes Glioblastoma Stem Cell Hypoxic Responses and Maintenance Through p53 Acetylation
Abstract
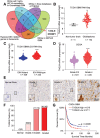
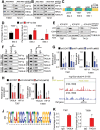
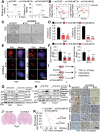
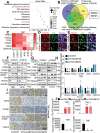
Glioblastoma (GBM) is a lethal cancer characterized by hypervascularity and necrosis associated with hypoxia. Here, it is found that hypoxia preferentially induces the actin-binding protein, Transgelin (TAGLN), in GBM stem cells (GSCs). Mechanistically, TAGLN regulates HIF1α transcription and stabilizes HDAC2 to deacetylate p53 and maintain GSC self-renewal. To translate these findings into preclinical therapeutic paradigm, it is found that sodium valproate (VPA) is a specific inhibitor of TAGLN/HDAC2 function, with augmented efficacy when combined with natural borneol (NB) in vivo. Thus, TAGLN promotes cancer stem cell survival in hypoxia and informs a novel therapeutic paradigm.
Keywords: HDAC2; HIF1α Hypoxia; glioblastoma stem cells; natural borneol; sodium valproate; transgelin.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- a) Janjua T. I., Rewatkar P., Ahmed‐Cox A., Saeed I., Mansfeld F. M., Kulshreshtha R., Kumeria T., Ziegler D. S., Kavallaris M., Mazzieri R., Popat A., Adv Drug Deliv Rev 2021, 171, 108; - PubMed
- b) Liu S., Shi W., Zhao Q., Zheng Z., Liu Z., Meng L., Dong L., Jiang X., Biomed. Pharmacother. 2021, 141, 111810. - PubMed
-
- a) Brennan C. W., Verhaak R. G. W., Mckenna A., Campos B., Noushmehr H., Salama S. R., Zheng S., Chakravarty D., Sanborn J. Z., Berman S. H., Beroukhim R., Bernard B., Wu C. J., Genovese G., Shmulevich I., Barnholtz‐Sloan J., Zou L., Vegesna R., Shukla S. A., Ciriello G., Yung W. K., Zhang W., Sougnez C., Mikkelsen T., Aldape K., Bigner D. D., Van Meir E. G., Prados M., Sloan A., Black K. L., et al., Cell 2013, 155, 462; - PMC - PubMed
- b) Parsons D. W., Jones S., Zhang X., Lin J. C.‐H., Leary R. J., Angenendt P., Mankoo P., Carter H., Siu I. M., Gallia G. L., Olivi A., Mclendon R., Rasheed B. A., Keir S., Nikolskaya T., Nikolsky Y., Busam D. A., Tekleab H., Diaz L. A., Hartigan J., Smith D. R., Strausberg R. L., Marie S. K. N., Shinjo S. M. O., Yan H., Riggins G. J., Bigner D. D., Karchin R., Papadopoulos N., Parmigiani G., et al., Science 2008, 321, 1807. - PMC - PubMed
-
- a) Obacz J., Pastorekova S., Vojtesek B., Hrstka R., Molecular Cancer 2013, 12; - PMC - PubMed
- b) Sermeus A., Michiels C., Cell Death Dis. 2011, 2, e164; - PMC - PubMed
- c) Koshikawa N., Maejima C., Miyazaki K., Nakagawara A., Takenaga K., Oncogene 2006, 25, 917; - PubMed
- d) Jing X., Yang F., Shao C., Wei K., Xie M., Shen H., Shu Y., Mol Cancer 2019, 18, 157; - PMC - PubMed
- e) Ashur‐Fabian O., Avivi A., Trakhtenbrot L., Adamsky K., Cohen M., Kajakaro G., Joel A., Amariglio N., Nevo E., Rechavi G., Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 12236. - PMC - PubMed
-
- a) Zhang Y., Dube C., Gibert M., Cruickshanks N., Wang B., Coughlan M., Yang Y., Setiady I., Deveau C., Saoud K., Grello C., Oxford M., Yuan F., Abounader R., Cancers, Basel 2018, 10, 297; - PMC - PubMed
- b) Wang L. B., Karpova A., Gritsenko M. A., Kyle J. E., Cao S., Li Y., Rykunov D., Colaprico A., Rothstein J. H., Hong R., Stathias V., Cornwell M., Petralia F., Wu Y., Reva B., Krug K., Pugliese P., Kawaler E., Olsen L. K., Liang W. W., Song X., Dou Y., Wendl M. C., Caravan W., Liu W., Cui Zhou D., Ji J., Tsai C. F., Petyuk V. A., Moon J., et al., Cancer Cell 2021, 34, 509; - PMC - PubMed
- c) Cancer N., Nature 2008, 455, 1061. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
