Predicting clinical progression trajectories of early Alzheimer's disease patients
- PMID: 38087949
- PMCID: PMC10984448
- DOI: 10.1002/alz.13565
Predicting clinical progression trajectories of early Alzheimer's disease patients
Abstract
Background: Models for forecasting individual clinical progression trajectories in early Alzheimer's disease (AD) are needed for optimizing clinical studies and patient monitoring.
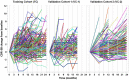
Methods: Prediction models were constructed using a clinical trial training cohort (TC; n = 934) via a gradient boosting algorithm and then evaluated in two validation cohorts (VC 1, n = 235; VC 2, n = 421). Model inputs included baseline clinical features (cognitive function assessments, APOE ε4 status, and demographics) and brain magnetic resonance imaging (MRI) measures.
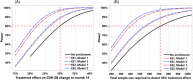
Results: The model using clinical features achieved R2 of 0.21 and 0.31 for predicting 2-year cognitive decline in VC 1 and VC 2, respectively. Adding MRI features improved the R2 to 0.29 in VC 1, which employed the same preprocessing pipeline as the TC. Utilizing these model-based predictions for clinical trial enrichment reduced the required sample size by 20% to 49%.
Discussion: Our validated prediction models enable baseline prediction of clinical progression trajectories in early AD, benefiting clinical trial enrichment and various applications.
Trial registration: ClinicalTrials.gov NCT02956486 NCT03036280 NCT01767311.
Keywords: clinical trial enrichment; disease progression; machine learning; mild cognitive impairment; prognosis.
© 2023 Eisai Inc. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
V.D., Y.Y., A.C., E.A., P.S., L.Z., H.H., L.K., S.D., and MI are employees of Eisai Inc. HH is also the Senior Associate Editor for the journal Alzheimer's & Dementia. No competing disclosures to report for DAL and LT. Author disclosures are available in the supporting information.
Figures




References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

