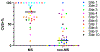A multicenter pilot study evaluating simplified central vein assessment for the diagnosis of multiple sclerosis
- PMID: 38088067
- PMCID: PMC11037932
- DOI: 10.1177/13524585231214360
A multicenter pilot study evaluating simplified central vein assessment for the diagnosis of multiple sclerosis
Abstract
Background: The central vein sign (CVS) is a proposed magnetic resonance imaging (MRI) biomarker for multiple sclerosis (MS); the optimal method for abbreviated CVS scoring is not yet established.
Objective: The aim of this study was to evaluate the performance of a simplified approach to CVS assessment in a multicenter study of patients being evaluated for suspected MS.
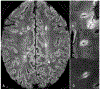
Methods: Adults referred for possible MS to 10 sites were recruited. A post-Gd 3D T2*-weighted MRI sequence (FLAIR*) was obtained in each subject. Trained raters at each site identified up to six CVS-positive lesions per FLAIR* scan. Diagnostic performance of CVS was evaluated for a diagnosis of MS which had been confirmed using the 2017 McDonald criteria at thresholds including three positive lesions (Select-3*) and six positive lesions (Select-6*). Inter-rater reliability assessments were performed.
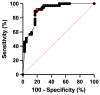
Results: Overall, 78 participants were analyzed; 37 (47%) were diagnosed with MS, and 41 (53%) were not. The mean age of participants was 45 (range: 19-64) years, and most were female (n = 55, 71%). The area under the receiver operating characteristic curve (AUROC) for the simplified counting method was 0.83 (95% CI: 0.73-0.93). Select-3* and Select-6* had sensitivity of 81% and 65% and specificity of 68% and 98%, respectively. Inter-rater agreement was 78% for Select-3* and 83% for Select-6*.
Conclusion: A simplified method for CVS assessment in patients referred for suspected MS demonstrated good diagnostic performance and inter-rater agreement.
Keywords: Central vein sign; FLAIR*; MRI; biomarkers; multiple sclerosis.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: L.D., C.M.O.D, M.A., J.D., E.C., P.Ra., and M.K.S. have declared no conflicts of interest. P.Ro. employed by and holds stocks in QMENTA. C.A. received consulting fees for scientific advisory boards for Genentech, EMD Serono, Alexion Pharmaceuticals, and Sanofi Genzyme. A.B.O. has received personal fees for advisory board participation and consulting from Abata, Accure, Atara Biotherapeutics, Biogen, BMS/Celgene/Receptos, GlaxoSmithKline, Gossamer, Horizon Therapeutics, Immunic, Janssen/Actelion, Medimmune, Merck/EMD Serono, Novartis, Roche/Genentech, Sangamo, Sanofi Genzyme, Viracta; and grant support to the University of Pennsylvania from Biogen Idec, Roche/Genentech, Merck/EMD Serono, and Novartis. P.A.C. is a PI on grants to JHU from Biogen and Annexon and served on scientific advisory boards for Disarm Therapeutics and Biogen. B.A.C.C. received compensation for consulting from Alexion, Atara, Biogen, EMD Serono, Novartis, Sanofi, and TG Therapeutics. L.F. received fees for consultancy and advisory board participation from Genentech, Novartis, Celgene/Bristol Myers Squibb, EMD Serono, and TG Therapeutics; received fees for educational activities from Medscape, LLC, and the MS Association of America; program sponsorship to UT from EMD Serono; and grant support to UT from NIH/NINDS, PCORI, Genentech, and EMD Serono. R.G.H. received research support from Roche, Genentech, Atara, and MedDay and consulting for Novartis, Sanofi/Genzyme, Roche/Genentech, QIA, and Neurona. E.E.L. received research support from Genentech, Biogen and consulting for Genentech, Janssen, TG Therapeutics, NGM Bio, Bristol Myers Squibb, and EMD Serono. J.O. received research support from Biogen Idec, Roche, and EMD Serono; consulting compensation from EMD Serono, Sanofi/Genzyme, Biogen Idec, Roche, Celgene, and Novartis. N.P. received research support from the Race to Erase MS Foundation. D.P. received consulting compensation from EMD Serono, Sanofi Genzyme, Roche, and Novartis. V.P. employed by and holds stocks in QMENTA. M.R. employed by and holds stock options in QMENTA. R.D.S. received fees for advisory board participation (Biogen, EMD Serono, Sanofi Genzyme) and consulting (EMD Serono, Biogen). E.S. received fees for scientific advisory boards and consulting for Alexion, Viela Bio, Horizon Therapeutics, Genentech, and Ad Scientiam; speaking honoraria from Alexion, Viela Bio, and Biogen. N.L.S. received research support from the National Institutes of Health, National Multiple Sclerosis Society, Patient-Centered Outcomes Research Institute, Race to Erase MS Foundation, and Biogen Idec. A.J.S. received fees for consulting: Greenwich Biosciences, Horizon Therapeutics, Kiniksa Pharmaceuticals, Octave Bioscience, TG Therapeutics; non-promotional speaking: EMD Serono; research funding: Bristol Myers Squibb; contracted research: Sanofi, Novartis, Actelion, Genentech/Roche. R.T.S. supported NIH R01NS112274, R01MH112847, and R01MH123550 and received consulting income from Octave Bioscience. D.S.R. supported by the Intramural Research Program of NINDS; received additional research support from Vertex Pharmaceuticals, Sanofi Genzyme, and Abata Therapeutics. P.S. received research support from the National Institutes of Health and the National Multiple Sclerosis Society. D.O. received research support from the National Institutes of Health, National Multiple Sclerosis Society, Patient-Centered Outcomes Research Institute, Race to Erase MS Foundation, Genentech, Genzyme, and Novartis; consulting fees from Biogen Idec, Genentech/Roche, Genzyme, Novartis, and Merck.
Figures