Effect of Ziltivekimab on Determinants of Hemoglobin in Patients with CKD Stage 3-5: An Analysis of a Randomized Trial (RESCUE)
- PMID: 38088558
- PMCID: PMC10786611
- DOI: 10.1681/ASN.0000000000000245
Effect of Ziltivekimab on Determinants of Hemoglobin in Patients with CKD Stage 3-5: An Analysis of a Randomized Trial (RESCUE)
Abstract
Significance statement: Systemic inflammation in CKD can lead to anemia. Ziltivekimab, a fully human monoclonal antibody targeting the IL-6 ligand, has been shown to reduce systemic inflammation in patients with CKD. It has also been shown to increase serum albumin in patients on hemodialysis with inflammation and hyporesponsiveness to treatment with erythropoiesis-stimulating agents. This exploratory analysis of the RESCUE clinical trial found that among patients with CKD stage 3-5 and systemic inflammation, ziltivekimab treatment significantly increased hemoglobin (Hb) levels after 12 weeks compared with placebo. Ziltivekimab was also associated with significant increases in serum iron levels, total iron-binding capacity, and transferrin saturation. No major safety concerns were reported. Further clinical trials are warranted to study ziltivekimab's potential for anemia management in patients with CKD.
Background: In the phase 2 RESCUE clinical trial, ziltivekimab, a fully human monoclonal antibody against the IL-6 ligand, significantly reduced the biomarkers of inflammation compared with placebo, in patients with CKD and systemic inflammation (high-sensitivity C-reactive protein ≥2 mg/L). The aim of this subanalysis of RESCUE trial data was to assess the effect of ziltivekimab on Hb and iron homeostasis in this patient population.
Methods: This was an analysis of exploratory end points from the RESCUE trial ( NCT03926117 ), which included 264 adults with CKD stage 3-5 and high-sensitivity C-reactive protein ≥2 mg/L. Participants received placebo or subcutaneous ziltivekimab (7.5, 15, or 30 mg) (1:1:1:1) once every 4 weeks, up to 24 weeks. End points for this analysis were changes in Hb and biomarkers of iron homeostasis from baseline to week 12.
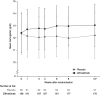
Results: The trial was terminated early due to the coronavirus disease 2019 pandemic, and thus, data up to week 12 are presented. Hb levels significantly increased from baseline to week 12 with ziltivekimab 7.5, 15, and 30 mg (treatment differences versus placebo: +0.57 g/dl [95% confidence interval, 0.27 to 0.86], +1.05 g/dl [0.76 to 1.33], and +0.99 g/dl [0.70 to 1.28], respectively, all P < 0.001). Ziltivekimab was associated with significant increases in serum iron levels, total iron-binding capacity, and transferrin saturation from baseline to week 12 ( P < 0.05 versus placebo for all doses and comparisons). Cases of sustained thrombocytopenia, sustained neutropenia, anemia, and iron deficiency anemia were infrequent and similar across all groups.
Conclusions: Anti-inflammatory therapy with ziltivekimab improved the markers of anemia and iron homeostasis in people with stage 3-5 CKD and systemic inflammation, suggesting a possible role in anemia management.
Trial registration: ClinicalTrials.gov NCT03926117 NCT02868229.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Society of Nephrology.
Conflict of interest statement
M. Davidson is former CEO and CMO of Corvidia Therapeutics, which was the sponsor of the RESCUE trial prior to acquisition by Novo Nordisk. C. Jensen reports Ownership Interest: Novo Nordisk A/S. M. Davidson reports: Employer: Chief Executive Officer of New Amsterdam Pharma, Professor of Medicine and Director of the Lipid Clinic at the University of Chicago; Consultancy: Novo Nordisk; Advisory or Leadership Role: Caladrius BioScience, Tenax Therapeutics; and Speakers Bureau: Esperion, Astra Zeneca, AMGEN, and Regeneron. C. Jensen, A.A. Mohseni Zonoozi, and P. Andreas Schytz are employees of Novo Nordisk A/S. P. Andreas Schytz also reports Ownership Interest: Novo Nordisk; and Research Funding: Novo Nordisk. P.E. Pergola was an investigator in the RESCUE trial and is a consultant for GSK and Novo Nordisk. P.E. Pergola also reports Employer: Renal Associates, P.A.; Consultancy: Ardelyx, AstraZeneca, Bayer, Renibus, Unicycive; Ownership Interest: Unicycive Therapeutics; Research Funding: Principal or subinvestigator on multiple clinical trials (contracts are with practice, not individual); and Advisory or Leadership Role: Ardelyx, Unicycive. V. Perkovic is employed by University of New South Wales Sydney and the Royal North Shore Hospital; and serves as a board director for St Vincent's Health Australia, George Clinical, and several medical research institutes. He has received honoraria for steering committee roles, scientific presentations, and/or advisory board attendance from AbbVie, Amgen, AstraZeneca, Baxter, Bayer, Boehringer Ingelheim, Chinook, Durect, Eli Lilly, Gilead, GSK, Janssen, Merck, Mitsubishi Tanabe Pharma Corporation, Mundipharma, Novartis, Novo Nordisk, Otsuka, Pfizer, PharmaLink, Reata, Relypsa, Roche, Sanofi, Servier, Travere, and Tricida. V. Perkovic also reports Consultancy: AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Mitsubishi Tanabe, Mundipharma, Novartis, Novo Nordisk, Otsuka, Travere, Tricida, UptoDate; Ownership Interest: George Clinical; Research Funding: AstraZeneca, Bayer, Chinook, Gilead, GlaxoSmithKline, Janssen, Novartis, Novo Nordisk, Otsuka, Travere, Tricida; and Honoraria: AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Mitsubishi Tanabe, Mundipharma, Novartis, Novo Nordisk, Otsuka, Travere, Tricida, UptoDate. D.S. Raj is supported by funding from the National Institutes of Health through 1 R01DK125256, R01 DK073665‐01A1, 1U01DK099924‐01, and 1U01DK099914‐01. He has received research funding from Relypsa and serves on the advisory board for Corvidia Therapeutics and Novo Nordisk. D.S. Raj also reports Consultancy: Novo Nordisk; Honoraria: Novo Nordisk; Advisory or Leadership Role: NHLBI; NIDDK; Novo Nordisk; and Other Interests or Relationships: American Association of Kidney Patients. K.R. Tuttle reports grants/contracts from the NIDDK/NIH, the NHLBI/NIH, the National Center for Advancing Translational Sciences (NCATS)/NIH, the Centers for Disease Control and Prevention (CDC) and Travere, and consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, Goldfinch Bio, Novo Nordisk, and Travere. She has also received honoraria from AstraZeneca, Bayer, Eli Lilly, Gilead, and Goldfinch Bio, and support for travel and meetings from Eli Lilly and Novo Nordisk. K.R. Tuttle is Chair of a data safety monitoring board for the NIDDK/NIH and the George Clinical Institute and is also Chair of the Diabetic Kidney Disease Collaborative Task Force of the American Society of Nephrology. She was on the Board of Directors for the Kidney Health Initiative of the US Food and Drug Administration and American Society of Nephrology. K.R. Tuttle also reports Research Funding: Bayer; and Honoraria: Boehringer Ingelheim, and Novo Nordisk.
Figures


References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

