Skin tropism during Usutu virus and West Nile virus infection: an amplifying and immunological role
- PMID: 38088560
- PMCID: PMC10805065
- DOI: 10.1128/jvi.01830-23
Skin tropism during Usutu virus and West Nile virus infection: an amplifying and immunological role
Abstract
Usutu virus (USUV) and West Nile virus (WNV) are closely related emerging arboviruses belonging to the Flavivirus genus and posing global public health concerns. Although human infection by these viruses is mainly asymptomatic, both have been associated with neurological disorders such as encephalitis and meningoencephalitis. Since USUV and WNV are transmitted through the bite of an infected mosquito, the skin represents the initial site of virus inoculation and provides the first line of host defense. Although some data on the early stages of WNV skin infection are available, very little is known about USUV. Herein, USUV-skin resident cell interactions were characterized. Using primary human keratinocytes and fibroblasts, an early replication of USUV during the first 24 hours was shown in both skin cells. In human skin explants, a high viral tropism for keratinocytes was observed. USUV infection of these models induced type I and III interferon responses associated with upregulated expression of various interferon-stimulated genes as well as pro-inflammatory cytokine and chemokine genes. Among the four USUV lineages studied, the Europe 2 strain replicated more efficiently in skin cells and induced a higher innate immune response. In vivo, USUV and WNV disseminated quickly from the inoculation site to distal cutaneous tissues. In addition, viral replication and persistence in skin cells were associated with an antiviral response. Taken together, these results provide a better understanding of the pathophysiology of the early steps of USUV infection and suggest that the skin constitutes a major amplifying organ for USUV and WNV infection.IMPORTANCEUsutu virus (USUV) and West Nile virus (WNV) are closely related emerging Flaviviruses transmitted through the bite of an infected mosquito. Since they are directly inoculated within the upper skin layers, the interactions between the virus and skin cells are critical in the pathophysiology of USUV and WNV infection. Here, during the early steps of infection, we showed that USUV can efficiently infect two human resident skin cell types at the inoculation site: the epidermal keratinocytes and the dermal fibroblasts, leading to the induction of an antiviral innate immune response. Moreover, following cutaneous inoculation, we demonstrated that both viruses can rapidly spread, replicate, and persist in all distal cutaneous tissues in mice, a phenomenon associated with a generalized skin inflammatory response. These results highlight the key amplifying and immunological role of the skin during USUV and WNV infection.
Keywords: Usutu virus; West Nile virus; fibroblasts; flavivirus; innate immune response; keratinocytes; skin tropism; viral dissemination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
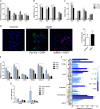
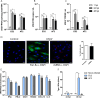
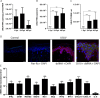
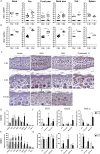
References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

