High fusion and cytopathy of SARS-CoV-2 variant B.1.640.1
- PMID: 38088562
- PMCID: PMC10805008
- DOI: 10.1128/jvi.01351-23
High fusion and cytopathy of SARS-CoV-2 variant B.1.640.1
Abstract
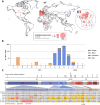
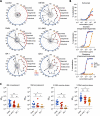
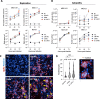
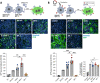
SARS-CoV-2 variants with undetermined properties have emerged intermittently throughout the COVID-19 pandemic. Some variants possess unique phenotypes and mutations which allow further characterization of viral evolution and Spike functions. Around 1,100 cases of the B.1.640.1 variant were reported in Africa and Europe between 2021 and 2022, before the expansion of Omicron. Here, we analyzed the biological properties of a B.1.640.1 isolate and its Spike. Compared to the ancestral Spike, B.1.640.1 carried 14 amino acid substitutions and deletions. B.1.640.1 escaped binding by some anti-N-terminal domain and anti-receptor-binding domain monoclonal antibodies, and neutralization by sera from convalescent and vaccinated individuals. In cell lines, infection generated large syncytia and a high cytopathic effect. In primary airway cells, B.1.640.1 replicated less than Omicron BA.1 and triggered more syncytia and cell death than other variants. The B.1.640.1 Spike was highly fusogenic when expressed alone. This was mediated by two poorly characterized and infrequent mutations located in the Spike S2 domain, T859N and D936H. Altogether, our results highlight the cytopathy of a hyper-fusogenic SARS-CoV-2 variant, supplanted upon the emergence of Omicron BA.1. (This study has been registered at ClinicalTrials.gov under registration no. NCT04750720.)IMPORTANCEOur results highlight the plasticity of SARS-CoV-2 Spike to generate highly fusogenic and cytopathic strains with the causative mutations being uncharacterized in previous variants. We describe mechanisms regulating the formation of syncytia and the subsequent consequences in a primary culture model, which are poorly understood.
Keywords: SARS-CoV-2; cytopathy; fusion; hNECs; syncytia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
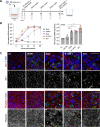
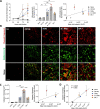

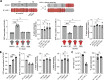
References
-
- Harvey WT, Carabelli AM, Jackson B, Gupta RK, Thomson EC, Harrison EM, Ludden C, Reeve R, Rambaut A, COVID-19 Genomics UK (COG-UK) Consortium, Peacock SJ, Robertson DL. 2021. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol 19:409–424. doi:10.1038/s41579-021-00573-0 - DOI - PMC - PubMed
-
- Planas D, Bruel T, Staropoli I, Guivel-Benhassine F, Porrot F, Maes P, Grzelak L, Prot M, Mougari S, Planchais C, Puech J, Saliba M, Sahraoui R, Fémy F, Morel N, Dufloo J, Sanjuán R, Mouquet H, André E, Hocqueloux L, Simon-Loriere E, Veyer D, Prazuck T, Péré H, Schwartz O. 2023. Resistance of Omicron subvariants BA.2.75.2, BA.4.6, and BQ.1.1 to neutralizing antibodies. Nat Commun 14:824. doi:10.1038/s41467-023-36561-6 - DOI - PMC - PubMed
-
- Bruel T, Stéfic K, Nguyen Y, Toniutti D, Staropoli I, Porrot F, Guivel-Benhassine F, Bolland WH, Planas D, Hadjadj J, Handala L, Planchais C, Prot M, Simon-Lorière E, André E, Baele G, Cuypers L, Mouthon L, Mouquet H, Buchrieser J, Sève A, Prazuck T, Maes P, Terrier B, Hocqueloux L, Schwartz O. 2022. Longitudinal analysis of serum neutralization of SARS-CoV-2 Omicron BA.2, BA.4, and BA.5 in patients receiving monoclonal antibodies. Cell Rep Med 3:100850. doi:10.1016/j.xcrm.2022.100850 - DOI - PMC - PubMed
-
- Willett BJ, Grove J, MacLean OA, Wilkie C, De Lorenzo G, Furnon W, Cantoni D, Scott S, Logan N, Ashraf S, et al. , PITCH Consortium, COVID-19 Genomics UK (COG-UK) Consortium . 2022. SARS-CoV-2 Omicron is an immune escape variant with an altered cell entry pathway. Nat Microbiol 7:1161–1179. doi:10.1038/s41564-022-01241-6 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

