2,2'-Bipyridine Derivatives as Halogen Bond Acceptors in Multicomponent Crystals
- PMID: 38089069
- PMCID: PMC10711937
- DOI: 10.1021/acs.cgd.3c01055
2,2'-Bipyridine Derivatives as Halogen Bond Acceptors in Multicomponent Crystals
Abstract
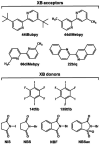
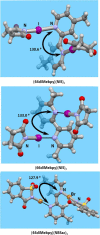
In this work, we present a systematic study of the halogen bonding potential of different 2,2'-bipyridine derivatives in the synthesis of cocrystals by using selected perfluorinated iodobenzenes and N-haloimides as halogen bond donors. These halogen bond acceptor molecules were chosen to explore how different substituents on 2,2'-bipyridine affect halogen bond formation. Out of 24 combinations, we obtained only 8 cocrystals by using two methods, liquid-assisted grinding and crystallization from the solution. Of those 8 cocrystals, one has already been described in the literature. As expected, structural data revealed that 2,2'-bipyridine derivatives act as ditopic halogen bond acceptors in all structures. Dominant interactions in 7 of the cocrystals are I···N or Br···N halogen bonds, while in the one remaining cocrystal it is the I···C(π) halogen bond.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Desiraju G. R.; Ho P. S.; Kloo L.; Legon A. C.; Marquardt R.; Metrangolo P.; Politzer P.; Resnati G.; Rissanen K. Definition of the halogen bond (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. 10.1351/PAC-REC-12-05-10. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
