Third-line or above anlotinib in relapsed and refractory small cell lung cancer patients with brain metastases: A post hoc analysis of ALTER1202, a randomized, double-blind phase 2 study
- PMID: 38089404
- PMCID: PMC10686170
- DOI: 10.1002/cai2.43
Third-line or above anlotinib in relapsed and refractory small cell lung cancer patients with brain metastases: A post hoc analysis of ALTER1202, a randomized, double-blind phase 2 study
Abstract
Background: The prognosis of patients with small cell lung cancer (SCLC) and brain metastases (BM) was poor. This study aimed to explore the efficacy and safety of anlotinib as third-line or above treatment in SCLC with BM.
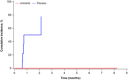
Methods: This was a subgroup analysis of the ALTER1202 trial, which was a randomized, placebo-controlled trial aimed to evaluate the role of anlotinib as third-line treatment or above in patients with SCLC. This study included patients with BM at baseline. The efficacy and safety outcomes included progression-free survival (PFS), overall survival (OS), central nervous system (CNS), objective response rate (ORR), CNS disease control rate (DCR), time to CNS progression, and adverse events (AEs).
Results: Twenty-one and nine patients with BM were included in the anlotinib and placebo groups, respectively. The median PFS and OS were 3.8 months (95% confidence interval [CI]: 1.8-6.1) and 6.1 months (95% CI: 4.1-8.0) in the anlotinib group. Anlotinib was associated with a significant improvement in PFS (hazard ratio [HR] = 0.15, 95% CI: 0.04-0.51, p = 0.0005) and OS (HR = 0.26, 95% CI: 0.09-0.73, p = 0.0061) than placebo. Anlotinib significantly prolonged the time to CNS progression (p < 0.0001). The anlotinib group had a higher CNS DCR than placebo (95.2% vs. 22.2%, p = 0.0001). The most common grade 3 or higher AEs were increased lipase (19.0%), hypertension (14.3%), and hyponatremia (14.3%) in the anlotinib group.
Conclusions: Anlotinib proved to have potential CNS activity and a manageable toxicity profile in patients with SCLC and BM, significantly delaying CNS progression.
Keywords: advanced small cell lung cancer; angiogenesis inhibitors; anlotinib; brain metastasis; safety.
© 2023 The Authors. Cancer Innovation published by John Wiley & Sons Ltd. on behalf of Tsinghua University Press.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
