The European Union One Health 2022 Zoonoses Report
- PMID: 38089471
- PMCID: PMC10714251
- DOI: 10.2903/j.efsa.2023.8442
The European Union One Health 2022 Zoonoses Report
Abstract
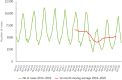
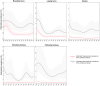
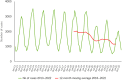
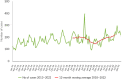
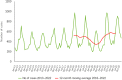
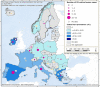
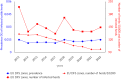
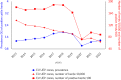
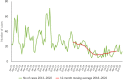
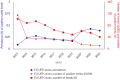
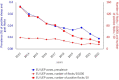
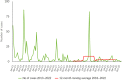
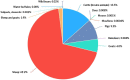
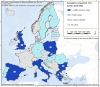
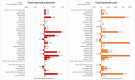
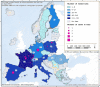
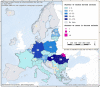
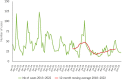
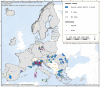
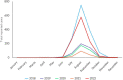
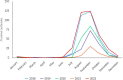
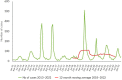
This report by the European Food Safety Authority and the European Centre for Disease Prevention and Control presents the results of the zoonoses monitoring and surveillance activities carried out in 2022 in 27 Member States (MSs), the United Kingdom (Northern Ireland) and 11 non-MSs. Key statistics on zoonoses and zoonotic agents in humans, food, animals and feed are provided and interpreted historically. In 2022, the first and second most reported zoonoses in humans were campylobacteriosis and salmonellosis, respectively. The number of cases of campylobacteriosis and salmonellosis remained stable in comparison with 2021. Nineteen MSs and the United Kingdom (Northern Ireland) achieved all the established targets in poultry populations for the reduction of Salmonella prevalence for the relevant serovars. Salmonella samples from carcases of various animal species, and samples for Campylobacter quantification from broiler carcases, were more frequently positive when performed by the competent authorities than when own checks were conducted. Yersiniosis was the third most reported zoonosis in humans, followed by Shiga toxin-producing Escherichia coli (STEC) and Listeria monocytogenes infections. L. monocytogenes and West Nile virus infections were the most severe zoonotic diseases, with the most hospitalisations and highest case fatality rates. In 2022, reporting showed an increase of more than 600% compared with 2021 in locally acquired cases of human West Nile virus infection, which is a mosquito-borne disease. In the EU, the number of reported foodborne outbreaks and cases, hospitalisations and deaths was higher in 2022 than in 2021. The number of deaths from outbreaks was the highest ever reported in the EU in the last 10 years, mainly caused by L. monocytogenes and to a lesser degree by Salmonella. Salmonella and in particular S. Enteritidis remained the most frequently reported causative agent for foodborne outbreaks. Norovirus (and other calicivirus) was the agent associated with the highest number of outbreak human cases. This report also provides updates on brucellosis, Coxiella burnetii (Q fever), echinococcosis, rabies, toxoplasmosis, trichinellosis, infection with Mycobacterium tuberculosis complex (focusing on Mycobacterium bovis and Mycobacterium caprae) and tularaemia.
Keywords: Campylobacter; Listeria; Salmonella; West Nile; foodborne outbreaks; monitoring; parasites; zoonoses.
© 2023 European Food Safety Authority and European Centre for Disease Prevention and Control.
Conflict of interest statement
If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contactinterestmanagement@efsa.europa.eu.
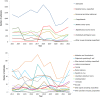
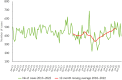
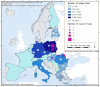
Figures







References
-
- Alba, P. , Carfora, V. , Feltrin, F. , Diaconu, E. L. , Sorbara, L. , Dell'Aira, E. , Cerci, T. , Ianzano, A. , Donati, V. , Franco, A. , & Battisti, A. (2023). Evidence of structural rearrangements in ESBL‐positive pESI(like) megaplasmids of S. Infantis . FEMS Microbiology Letters, 370, 1–8. 10.1093/femsle/fnad014 - DOI - PMC - PubMed
-
- Alban, L. , Pozio, E. , Boes, J. , Boireau, P. , Boué, F. , Claes, M. , Cook, A. J. , Dorny, P. , Enemark, H. L. , van der Giessen, J. , Hunt, K. R. , Howell, M. , Kirjusina, M. , Nöckler, K. , Rossi, P. , Smith, G. C. , Snow, L. , Taylor, M. A. , Theodoropoulos, G. , … Zimmer, I. A. (2011). Towards a standardised surveillance for Trichinella in the European Union. Preventive Veterinary Medicine, 99(2–4), 148–160. 10.1016/j.prevetmed.2011.02.008 - DOI - PubMed
-
- Aroussi, A. , Vignoles, P. , Dalmay, F. , Wimel, L. , Dardé, M. L. , Mercier, A. , & Ajzenberg, D. (2015). Detection of toxoplasma gondii DNA in horse meat from supermarkets in France and performance evaluation of two serological tests. Parasite, 22, 14. 10.1051/parasite/2015014 - DOI - PMC - PubMed
-
- Balen Topić, M. , Papić, N. , Višković, K. , Sviben, M. , Filipec Kanižaj, T. , Jadrijević, S. , Jurković, D. , & Beck, R. (2023). Emergence of Echinococcus multilocularis in central continental Croatia: A human case series and update on prevalence in foxes. Life (Basel), 13(6), 1402. 10.3390/life13061402 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
