Latest progress in low-intensity pulsed ultrasound for studying exosomes derived from stem/progenitor cells
- PMID: 38089611
- PMCID: PMC10715436
- DOI: 10.3389/fendo.2023.1286900
Latest progress in low-intensity pulsed ultrasound for studying exosomes derived from stem/progenitor cells
Abstract
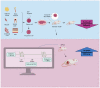
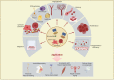
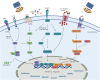
Stem cells have self-renewal, replication, and multidirectional differentiation potential, while progenitor cells are undifferentiated, pluripotent or specialized stem cells. Stem/progenitor cells secrete various factors, such as cytokines, exosomes, non-coding RNAs, and proteins, and have a wide range of applications in regenerative medicine. However, therapies based on stem cells and their secreted exosomes present limitations, such as insufficient source materials, mature differentiation, and low transplantation success rates, and methods addressing these problems are urgently required. Ultrasound is gaining increasing attention as an emerging technology. Low-intensity pulsed ultrasound (LIPUS) has mechanical, thermal, and cavitation effects and produces vibrational stimuli that can lead to a series of biochemical changes in organs, tissues, and cells, such as the release of extracellular bodies, cytokines, and other signals. These changes can alter the cellular microenvironment and affect biological behaviors, such as cell differentiation and proliferation. Here, we discuss the effects of LIPUS on the biological functions of stem/progenitor cells, exosomes, and non-coding RNAs, alterations involved in related pathways, various emerging applications, and future perspectives. We review the roles and mechanisms of LIPUS in stem/progenitor cells and exosomes with the aim of providing a deeper understanding of LIPUS and promoting research and development in this field.
Keywords: differentiation; exosomes; homing; low-intensity pulsed ultrasound; migration; proliferation; stem cells.
Copyright © 2023 He, Wang, Deng, Wang, Huang, Lin and Lyu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Advances in the application of low-intensity pulsed ultrasound to mesenchymal stem cells.Stem Cell Res Ther. 2022 May 26;13(1):214. doi: 10.1186/s13287-022-02887-z. Stem Cell Res Ther. 2022. PMID: 35619156 Free PMC article. Review.
-
Low-intensity pulsed ultrasound stimulates proliferation of stem/progenitor cells: what we need to know to translate basic science research into clinical applications.Asian J Androl. 2021 Nov-Dec;23(6):602-610. doi: 10.4103/aja.aja_25_21. Asian J Androl. 2021. PMID: 33818526 Free PMC article. Review.
-
Jingle Cell Rock: Steering Cellular Activity With Low-Intensity Pulsed Ultrasound (LIPUS) to Engineer Functional Tissues in Regenerative Medicine.Ultrasound Med Biol. 2024 Dec;50(12):1973-1986. doi: 10.1016/j.ultrasmedbio.2024.08.016. Epub 2024 Sep 16. Ultrasound Med Biol. 2024. PMID: 39289118 Review.
-
Application of low-intensity pulsed ultrasound on tissue resident stem cells: Potential for ophthalmic diseases.Front Endocrinol (Lausanne). 2023 Mar 17;14:1153793. doi: 10.3389/fendo.2023.1153793. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37008913 Free PMC article. Review.
-
Impact of Ultrasound Therapy on Stem Cell Differentiation - A Systematic Review.Curr Stem Cell Res Ther. 2020;15(5):462-472. doi: 10.2174/1574888X15666200225124934. Curr Stem Cell Res Ther. 2020. PMID: 32096749
Cited by
-
Time-Dependent Effects of Low-Intensity Pulsed Ultrasound on Apoptosis and Autophagy in Malignant Melanoma Stem Cells.J Cell Mol Med. 2025 Jun;29(12):e70687. doi: 10.1111/jcmm.70687. J Cell Mol Med. 2025. PMID: 40561182 Free PMC article.
-
Exploration of the optimal retention method in vivo for stem cell therapy: Low-intensity ultrasound preconditioning.Regen Ther. 2025 Apr 30;29:484-492. doi: 10.1016/j.reth.2025.04.012. eCollection 2025 Jun. Regen Ther. 2025. PMID: 40390863 Free PMC article.
-
Exosomes as Promising Therapeutic Tools for Regenerative Endodontic Therapy.Biomolecules. 2024 Mar 11;14(3):330. doi: 10.3390/biom14030330. Biomolecules. 2024. PMID: 38540750 Free PMC article. Review.
-
Piezoelectricity of hexagonal boron nitrides improves bone tissue generation as tested on osteoblasts.Beilstein J Nanotechnol. 2025 Jul 7;16:1068-1081. doi: 10.3762/bjnano.16.78. eCollection 2025. Beilstein J Nanotechnol. 2025. PMID: 40661707 Free PMC article.
References
-
- Navas A, Magaña-Guerrero FS, Domínguez-López A, Chávez-García C, Partido G, Graue-Hernández EO, et al. . Anti-inflammatory and anti-fibrotic effects of human amniotic membrane mesenchymal stem cells and their potential in corneal repair. Stem Cells Transl Med (2018) 7(12):906–17. doi: 10.1002/sctm.18-0042 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

