Phase Segregation Mechanisms in Mixed-Halide CsPb(BrxI1- x)3 Nanocrystals in Dependence of Their Sizes and Their Initial [Br]:[I] Ratios
- PMID: 38089654
- PMCID: PMC10636778
- DOI: 10.1021/acsmaterialsau.3c00056
Phase Segregation Mechanisms in Mixed-Halide CsPb(BrxI1- x)3 Nanocrystals in Dependence of Their Sizes and Their Initial [Br]:[I] Ratios
Abstract
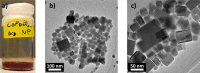
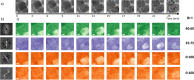
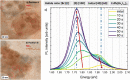
Phase segregation in inorganic CsPb(BrxI1-x)3 nanoparticles (NPs) exhibiting originally a homogeneous [Br]:[I] mixture was investigated by means of in situ transmission electron microscopy (TEM) and evaluated by using multivariate analyses. The colloidal synthesis of the NPs offers good control of the halide ratios on the nanoscale. The spatially resolved TEM investigations were correlated with integral photoluminescence measurements. By this approach, the halide-segregation processes and their spatial distributions can be described as being governed by the interaction of three partial processes: electron- and photon-irradiation-induced iodide oxidation, local differences in band gap energy, and intrinsic lattice strain. Since the oxidation can be induced by both electron-beam and light irradiation, both irradiation types can induce phase segregation in CsPb(BrxI1-x)3 compounds. This makes in situ TEM a valuable tool to monitor phase transformation in corresponding NPs and thin films on the sub-nm scale.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- NREL . Interactive Best Research-Cell Efficiency Chart, https://www.nrel.gov/pv/interactive-cell-efficiency.html (accessed Aug 28, 2023).
-
- Al-Ashouri A.; Köhnen E.; Li B.; Magomedov A.; Hempel H.; Caprioglio P.; Márquez J. A.; Morales Vilches A. B.; Kasparavicius E.; Smith J. A.; Phung N.; Menzel D.; Grischek M.; Kegelmann L.; Skroblin D.; Gollwitzer C.; Malinauskas T.; Jošt M.; Matič G.; Rech B.; Schlatmann R.; Topič M.; Korte L.; Abate A.; Stannowski B.; Neher D.; Stolterfoht M.; Unold T.; Getautis V.; Albrecht S. Monolithic Perovskite/Silicon Tandem Solar Cell with >29% Efficiency by Enhanced Hole Extraction. Science 2020, 370, 1300–1309. 10.1126/science.abd4016. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
