Erythromycin, retapamulin, pyridoxine, folic acid, and ivermectin inhibit cytopathic effect, papain-like protease, and MPRO enzymes of SARS-CoV-2
- PMID: 38089816
- PMCID: PMC10711598
- DOI: 10.3389/fcimb.2023.1273982
Erythromycin, retapamulin, pyridoxine, folic acid, and ivermectin inhibit cytopathic effect, papain-like protease, and MPRO enzymes of SARS-CoV-2
Abstract
Background: Although tremendous success has been achieved in the development and deployment of effective COVID-19 vaccines, developing effective therapeutics for the treatment of those who do come down with the disease has been with limited success. To repurpose existing drugs for COVID-19, we previously showed, qualitatively, that erythromycin, retapamulin, pyridoxine, folic acid, and ivermectin inhibit SARS-COV-2-induced cytopathic effect (CPE) in Vero cells.
Aim: This study aimed to quantitatively explore the inhibition of SARS-CoV-2-induced CPE by erythromycin, retapamulin, pyridoxine, folic acid, and ivermectin and to determine the effect of these drugs on SARS-CoV-2 papain-like protease and 3CL protease (MPRO) enzymes.
Methods: Neutral red (3-amino-7-dimethylamino-2-methyl-phenazine hydrochloride) cell viability assay was used to quantify CPE after infecting pre-treated Vero cells with clinical SARS-Cov-2 isolates. Furthermore, SensoLyte® 520 SARS-CoV-2 papain-like protease and SensoLyte® 520 SARS-CoV-2 MPRO activity assay kits were used to evaluate the inhibitory activity of the drugs on the respective enzymes.
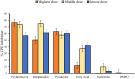
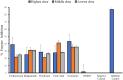
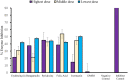
Results: Erythromycin, retapamulin, pyridoxine, folic acid, and ivermectin dose-dependently inhibit SARS-CoV-2-induced CPE in Vero cells, with inhibitory concentration-50 (IC50) values of 3.27 µM, 4.23 µM, 9.29 µM, 3.19 µM, and 84.31 µM, respectively. Furthermore, erythromycin, retapamulin, pyridoxine, folic acid, and ivermectin dose-dependently inhibited SARS-CoV-2 papain-like protease with IC50 values of 0.94 µM, 0.88 µM, 1.14 µM, 1.07 µM, and 1.51 µM, respectively, and inhibited the main protease (MPRO) with IC50 values of 1.35 µM, 1.25 µM, 7.36 µM, 1.15 µM, and 2.44 µM, respectively.
Conclusion: The IC50 for all the drugs, except ivermectin, was at the clinically achievable plasma concentration in humans, which supports a possible role for the drugs in the management of COVID-19. The lack of inhibition of CPE by ivermectin at clinical concentrations could be part of the explanation for its lack of effectiveness in clinical trials.
Keywords: COVID-19; SARS-CoV-2; erythromycin; folic acid; ivermectin; pyridoxine; retapamulin.
Copyright © 2023 Bello, Imam, Bello, Yunusa, Ahmed Adamu, Shuaibu, Igumbor, Habib, Popoola, Ochu, Yahaya Bello, Deeni and Okoye.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Beigmohammadi M., Bitarafan S., Hoseindokht A., Abdollahi A., Amoozadeh L., Mahmoodi Ali Abadi M., et al. . (2020). ‘Impact of vitamins A, B, C, D, and e supplementation on improvement and mortality rate in ICU patients with coronavirus-19: A structured summary of a study protocol for a randomized controlled trial. Trials 21 (1), 614. doi: 10.1186/s13063-020-04547-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

