Potential Causes of Spread of Antimicrobial Resistance and Preventive Measures in One Health Perspective-A Review
- PMID: 38089962
- PMCID: PMC10715026
- DOI: 10.2147/IDR.S428837
Potential Causes of Spread of Antimicrobial Resistance and Preventive Measures in One Health Perspective-A Review
Abstract
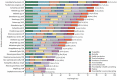
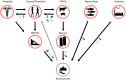
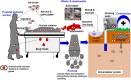
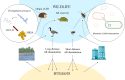
Antimicrobial resistance, referring to microorganisms' capability to subsist and proliferate even when there are antimicrobials is a foremost threat to public health globally. The appearance of antimicrobial resistance can be ascribed to anthropological, animal, and environmental factors. Human-related causes include antimicrobial overuse and misuse in medicine, antibiotic-containing cosmetics and biocides utilization, and inadequate sanitation and hygiene in public settings. Prophylactic and therapeutic antimicrobial misuse and overuse, using antimicrobials as feed additives, microbes resistant to antibiotics and resistance genes in animal excreta, and antimicrobial residue found in animal-origin food and excreta are animals related contributive factors for the antibiotic resistance emergence and spread. Environmental factors including naturally existing resistance genes, improper disposal of unused antimicrobials, contamination from waste in public settings, animal farms, and pharmaceutical industries, and the use of agricultural and sanitation chemicals facilitatet its emergence and spread. Wildlife has a plausible role in the antimicrobial resistance spread. Adopting a one-health approach involving using antimicrobials properly in animals and humans, improving sanitation in public spaces and farms, and implementing coordinated governmental regulations is crucial for combating antimicrobial resistance. Collaborative and cooperative involvement of stakeholders in public, veterinary and ecological health sectors is foremost to circumvent the problem effectively.
Keywords: animal; antimicrobial resistance; antimicrobial resistance gene; environment; one health; wildlife.
© 2023 Endale et al.
Conflict of interest statement
All authors declared that there is no conflict of interest upon publication of this manuscript.
Figures





References
-
- WHO. Antimicrobial Resistance; 2021.
Publication types
LinkOut - more resources
Full Text Sources

