Kamishoyosan and Kamikihito protect against decreased KCC2 expression induced by the P. gingivalis lipopolysaccharide treatment in PC-12 cells and improve behavioral abnormalities in male mice
- PMID: 38090003
- PMCID: PMC10711140
- DOI: 10.1016/j.heliyon.2023.e22784
Kamishoyosan and Kamikihito protect against decreased KCC2 expression induced by the P. gingivalis lipopolysaccharide treatment in PC-12 cells and improve behavioral abnormalities in male mice
Abstract
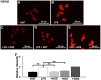
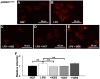
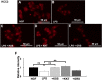
Kamishoyosan (KSS) and Kamikihito (KKT) have been traditionally prescribed for neuropsychiatric symptoms in Japan. However, the molecular mechanism of its effect is not elucidated enough. On the other hand, it has been reported that lipopolysaccharide derived from Porphyromonas gingivalis (P. g LPS) is involved not only in periodontal disease but also in the systemic diseases such as psychiatric disorders via neuroinflammation. Here, we investigated the molecular mechanism of KSS and KKT treatment by LPS-induced neuropathy using PC-12 cells. When P. g LPS was administrated during the NGF treatment, the KCC2 expression was decreased in PC-12 cells. P. g LPS treatment also decreased the WNK and phospho SPAK (pSPAK) expression and enhanced GSK-3β expression that negatively regulates WNK-SPAK signaling. Moreover, when KSS or KKT was administrated before P. g LPS treatment, the decrease of KCC2, WNK and pSPAK was rescued. KSS and KKT treatment also rescued the enhancement of GSK3β expression by P. g LPS treatment. Furthermore, KSS, KKT and/or oxytocin could rescue behavioral abnormalities caused by P. g LPS treatment by animal experiments. These effects were not shown in the Goreisan treatment, which has been reported to act on the central nervous system. These results indicate that KSS and KKT are candidates for therapeutic agents for neural dysfunction.
Keywords: GABA; KCC2; LPS; Oxytocin; PC-12 cells.
© 2023 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





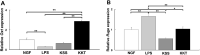



Similar articles
-
Therapeutic potential for KCC2-targeted neurological diseases.Jpn Dent Sci Rev. 2023 Dec;59:431-438. doi: 10.1016/j.jdsr.2023.11.001. Epub 2023 Nov 11. Jpn Dent Sci Rev. 2023. PMID: 38022385 Free PMC article. Review.
-
Oxytocin ameliorates KCC2 decrease induced by oral bacteria-derived LPS that affect rat primary cultured cells and PC-12 cells.Peptides. 2022 Apr;150:170734. doi: 10.1016/j.peptides.2021.170734. Epub 2021 Dec 31. Peptides. 2022. PMID: 34974081
-
Effect of Japanese Kampo Medicine Therapy for Menopausal Symptoms after Treatment of Gynecological Malignancy.Obstet Gynecol Int. 2018 Apr 2;2018:9475919. doi: 10.1155/2018/9475919. eCollection 2018. Obstet Gynecol Int. 2018. PMID: 29805451 Free PMC article.
-
Kamikihito Ameliorates Lipopolysaccharide-Induced Sickness Behavior via Attenuating Neural Activation, but Not Inflammation, in the Hypothalamic Paraventricular Nucleus and Central Nucleus of the Amygdala in Mice.Biol Pharm Bull. 2016;39(2):289-94. doi: 10.1248/bpb.b15-00707. Biol Pharm Bull. 2016. PMID: 26830488
-
Effects of SPAK knockout on sensorimotor gating, novelty exploration, and brain area-dependent expressions of NKCC1 and KCC2 in a mouse model of schizophrenia.Prog Neuropsychopharmacol Biol Psychiatry. 2015 Aug 3;61:30-6. doi: 10.1016/j.pnpbp.2015.03.007. Epub 2015 Mar 19. Prog Neuropsychopharmacol Biol Psychiatry. 2015. PMID: 25797415 Review.
Cited by
-
Therapeutic potential for KCC2-targeted neurological diseases.Jpn Dent Sci Rev. 2023 Dec;59:431-438. doi: 10.1016/j.jdsr.2023.11.001. Epub 2023 Nov 11. Jpn Dent Sci Rev. 2023. PMID: 38022385 Free PMC article. Review.
References
-
- Matsuda K. The effect of KAMISHOYOSAN on two male cases of vegetative stigmata. J. Japan Soc. Orient. Med. 1975;26:158–160. doi: 10.14868/kampomed1950.26.158. - DOI
-
- Lee J.Y., Oh H.K., Ryu H.S., Yoon S.S., Eo W., Yoon S.W. Efficacy and safety of the traditional herbal medicine, Gamiguibi-tang, in patients with cancer-related sleep disturbance: a prospective, randomized, wait-list-controlled, pilot study. Integr. Cancer Ther. 2018;17:524–530. doi: 10.1177/1534735417734914xd. - DOI - PMC - PubMed
-
- Igarashi K., Kuchiiwa T., Kuchiiwa S., Iwai H., Tomita K., Kamishoyosan T. Sato. (a Japanese traditional herbal formula), which effectively reduces the aggressive biting behavior of male and female mice, and potential regulation through increase of Tph1, Tph2, and Esr2 mRNA levels. Brain Res. 2021;1768 - PubMed
-
- Tsukada M., Ikemoto H., Lee X.P., Takaki T., Tsuchiya N., Mizuno K., Inoue T., Tsunokawa Y., Okumo T., Matsuyama T., Kamikihito M. Sunagawa. A traditional Japanese Kampo medicine, increases the secretion of oxytocin in rats with acute stress. J. Ethnopharmacol. 2021;276 doi: 10.1016/j.jep.2021.114218. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

