Chalcone Mannich base derivatives: synthesis, antimalarial activities against Plasmodium knowlesi, and molecular docking analysis
- PMID: 38090066
- PMCID: PMC10714133
- DOI: 10.1039/d3ra05361j
Chalcone Mannich base derivatives: synthesis, antimalarial activities against Plasmodium knowlesi, and molecular docking analysis
Abstract
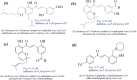
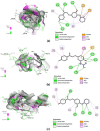
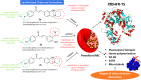
Development and discovery of new antimalarial drugs are needed to overcome the multi-resistance of Plasmodium parasites to commercially available drugs. Modifying the substitutions on the amine groups has been shown to increase antimalarial activities and decrease cross-resistance with chloroquine. In this study, we have synthesized several chalcone derivatives via the substitution of aminoalkyl groups into the aromatic chalcone ring using the Mannich-type reaction. The chalcone derivatives were evaluated for their antimalarial properties against Plasmodium knowlesi A1H1 and P. falciparum 3D7, as well as their molecular docking on Plasmodium falciparum dihydrofolate reductases-thymidylate synthase (PfDHFR-TS). Data from in vitro evaluation showed that chalcone Mannich-type base derivatives 2a, 2e, and 2h displayed potential antimalarial activities against P. knowlesi with EC50 of 2.64, 2.98, and 0.10 μM, respectively, and P. falciparum 3D7 with EC50 of 0.08, 2.69, and 0.15 μM, respectively. The synthesized compounds 2a, 2e, and 2h exerted high selectivity index (SI > 10) values on the A1H1 and 3D7 strains. The molecular docking analysis on PfDHFR-TS supported the in vitro assay of 2a, 2e, and 2h by displaying CDOCKER energy of -48.224, -43.292, and -45.851 kcal mol-1. Therefore, the evidence obtained here supports that PfDHFR-TS is a putative molecular target for the synthesized compound.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- World Health Organization (WHO), World Malaria Report, Geneva, 2022, https://www.who.int/teams/global-malaria-programme/reports/world-malaria...
-
- Ministry of Health (MOH), Tren Kasus Malaria Menurun, Indonesia, 2022, https://www.kemkes.go.id/article/view/21042300004/tren-kasus-malaria-men..., accessed on 30 June 2022
-
- Sibley C. H. Science. 2015;347:373–374. - PubMed
LinkOut - more resources
Full Text Sources

