Clinical course of pathologically confirmed corticobasal degeneration and corticobasal syndrome
- PMID: 38090279
- PMCID: PMC10715783
- DOI: 10.1093/braincomms/fcad296
Clinical course of pathologically confirmed corticobasal degeneration and corticobasal syndrome
Erratum in
-
Correction to: Clinical course of pathologically confirmed corticobasal degeneration and corticobasal syndrome.Brain Commun. 2024 Feb 12;6(1):fcae028. doi: 10.1093/braincomms/fcae028. eCollection 2024. Brain Commun. 2024. PMID: 38347942 Free PMC article.
Abstract
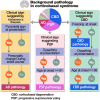
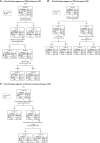
The clinical presentation of corticobasal degeneration is diverse, while the background pathology of corticobasal syndrome is also heterogeneous. Therefore, predicting the pathological background of corticobasal syndrome is extremely difficult. Herein, we investigated the clinical findings and course in patients with pathologically, genetically and biochemically verified corticobasal degeneration and corticobasal syndrome with background pathology to determine findings suggestive of background disorder. Thirty-two patients were identified as having corticobasal degeneration. The median intervals from the initial symptoms to the onset of key milestones were as follows: gait disturbance, 0.0 year; behavioural changes, 1.0 year; falls, 2.0 years; cognitive impairment, 2.0 years; speech impairment, 2.5 years; supranuclear gaze palsy, 3.0 years; urinary incontinence, 3.0 years; and dysphagia, 5.0 years. The median survival time was 7.0 years; 50% of corticobasal degeneration was diagnosed as corticobasal degeneration/corticobasal syndrome at the final presentation. Background pathologies of corticobasal syndrome (n = 48) included corticobasal degeneration (33.3%), progressive supranuclear palsy (29.2%) and Alzheimer's disease (12.5%). The common course of corticobasal syndrome was initial gait disturbance and early fall. In addition, corticobasal degeneration-corticobasal syndrome manifested behavioural change (2.5 years) and cognitive impairment (3.0 years), as the patient with progressive supranuclear palsy-corticobasal syndrome developed speech impairment (1.0 years) and supranuclear gaze palsy (6.0 years). The Alzheimer's disease-corticobasal syndrome patients showed cognitive impairment (1.0 years). The frequency of frozen gait at onset was higher in the corticobasal degeneration-corticobasal syndrome group than in the progressive supranuclear palsy-corticobasal syndrome group [P = 0.005, odds ratio (95% confidence interval): 31.67 (1.46-685.34)]. Dysarthria at presentation was higher in progressive supranuclear palsy-corticobasal syndrome than in corticobasal degeneration-corticobasal syndrome [P = 0.047, 6.75 (1.16-39.20)]. Pyramidal sign at presentation and personality change during the entire course were higher in Alzheimer's disease-corticobasal syndrome than in progressive supranuclear palsy-corticobasal syndrome [P = 0.011, 27.44 (1.25-601.61), and P = 0.013, 40.00 (1.98-807.14), respectively]. In corticobasal syndrome, decision tree analysis revealed that 'freezing at onset' or 'no dysarthria at presentation and age at onset under 66 years in the case without freezing at onset' predicted corticobasal degeneration pathology with a sensitivity of 81.3% and specificity of 84.4%. 'Dysarthria at presentation and age at onset over 61 years' suggested progressive supranuclear palsy pathology, and 'pyramidal sign at presentation and personality change during the entire course' implied Alzheimer's disease pathology. In conclusion, frozen gait at onset, dysarthria, personality change and pyramidal signs may be useful clinical signs for predicting background pathologies in corticobasal syndrome.
Keywords: clinical course; corticobasal degeneration; corticobasal syndrome; diagnosis; pathology.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures




Comment in
-
Correction to: Clinical course of pathologically confirmed corticobasal degeneration and corticobasal syndrome.Brain Commun. 2024 Feb 12;6(1):fcae028. doi: 10.1093/braincomms/fcae028. eCollection 2024. Brain Commun. 2024. PMID: 38347942 Free PMC article.
References
-
- Rebeiz JJ, Kolodny EH, Richardson EP Jr. Corticodentatonigral degeneration with neuronal achromasia. Arch Neurol. 1968;18(1):20–33. - PubMed
-
- Dickson DW, Bergeron C, Chin SS, et al. Office of Rare Diseases of the National Institutes of Health. Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol. 2002;61(11):935–946. - PubMed
-
- Kovacs GG. Invited review: Neuropathology of tauopathies: Principles and practice. Neuropathol Appl Neurobiol. 2015;41(1):3–23. - PubMed
LinkOut - more resources
Full Text Sources
